Related Research Articles

An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

Immunoglobulin G (IgG) is a type of antibody. Representing approximately 75% of serum antibodies in humans, IgG is the most common type of antibody found in blood circulation. IgG molecules are created and released by plasma B cells. Each IgG antibody has two paratopes.

Immunoglobulin A is an antibody that plays a role in the immune function of mucous membranes. The amount of IgA produced in association with mucosal membranes is greater than all other types of antibody combined. In absolute terms, between three and five grams are secreted into the intestinal lumen each day. This represents up to 15% of total immunoglobulins produced throughout the body.

Immunoglobulin D (IgD) is an antibody isotype that makes up about 1% of proteins in the plasma membranes of immature B-lymphocytes where it is usually co-expressed with another cell surface antibody called IgM. IgD is also produced in a secreted form that is found in very small amounts in blood serum, representing 0.25% of immunoglobulins in serum. The relative molecular mass and half-life of secreted IgD is 185 kDa and 2.8 days, respectively. Secreted IgD is produced as a monomeric antibody with two heavy chains of the delta (δ) class, and two Ig light chains.
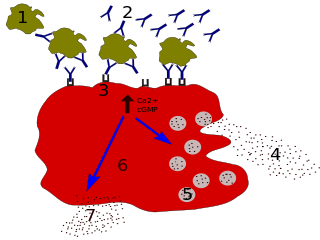
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.

Immunoglobulin M (IgM) is one of several isotypes of antibody that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antigen. that's why it is also called acute phase antibody.In humans and other mammals that have been studied, plasmablasts residing in the spleen are the main source of specific IgM production.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.

In immunology, a Fc receptor is a protein found on the surface of certain cells – including, among others, B lymphocytes, follicular dendritic cells, natural killer cells, macrophages, neutrophils, eosinophils, basophils, human platelets, and mast cells – that contribute to the protective functions of the immune system. Its name is derived from its binding specificity for a part of an antibody known as the Fc region. Fc receptors bind to antibodies that are attached to infected cells or invading pathogens. Their activity stimulates phagocytic or cytotoxic cells to destroy microbes, or infected cells by antibody-mediated phagocytosis or antibody-dependent cell-mediated cytotoxicity. Some viruses such as flaviviruses use Fc receptors to help them infect cells, by a mechanism known as antibody-dependent enhancement of infection.

Protein A is a 42 kDa surface protein originally found in the cell wall of the bacteria Staphylococcus aureus. It is encoded by the spa gene and its regulation is controlled by DNA topology, cellular osmolarity, and a two-component system called ArlS-ArlR. It has found use in biochemical research because of its ability to bind immunoglobulins. It is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs. It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of the human VH3 family. Through these interactions in serum, where IgG molecules are bound in the wrong orientation, the bacteria disrupts opsonization and phagocytosis.
CD16, also known as FcγRIII, is a cluster of differentiation molecule found on the surface of natural killer cells, neutrophils, monocytes, macrophages, and certain T cells. CD16 has been identified as Fc receptors FcγRIIIa (CD16a) and FcγRIIIb (CD16b), which participate in signal transduction. The most well-researched membrane receptor implicated in triggering lysis by NK cells, CD16 is a molecule of the immunoglobulin superfamily (IgSF) involved in antibody-dependent cellular cytotoxicity (ADCC). It can be used to isolate populations of specific immune cells through fluorescent-activated cell sorting (FACS) or magnetic-activated cell sorting, using antibodies directed towards CD16.

In immunology, antibodies are classified into several types called isotypes or classes. The variable (V) regions near the tip of the antibody can differ from molecule to molecule in countless ways, allowing it to specifically target an antigen . In contrast, the constant (C) regions only occur in a few variants, which define the antibody's class. Antibodies of different classes activate distinct effector mechanisms in response to an antigen . They appear at different stages of an immune response, differ in structural features, and in their location around the body.
Protein A/G is a recombinant fusion protein that combines IgG binding domains of both Protein A and Protein G. Protein A/G contains four Fc binding domains from Protein A and two from Protein G, yielding a final mass of 50,460 daltons. The binding of Protein A/G is less pH-dependent than Protein A, but otherwise has the additive properties of Protein A and G.

Leukocyte immunoglobulin-like receptor subfamily B member 1 is a protein that in humans is encoded by the LILRB1 gene.

Leukocyte immunoglobulin-like receptor subfamily B member 2 is a protein that in humans is encoded by the LILRB2 gene.
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.

Fc fragment of IgA receptor (FCAR) is a human gene that codes for the transmembrane receptor FcαRI, also known as CD89. FcαRI binds the heavy-chain constant region of Immunoglobulin A (IgA) antibodies. FcαRI is present on the cell surface of myeloid lineage cells, including neutrophils, monocytes, macrophages, and eosinophils, though it is notably absent from intestinal macrophages and does not appear on mast cells. FcαRI plays a role in both pro- and anti-inflammatory responses depending on the state of IgA bound. Inside-out signaling primes FcαRI in order for it to bind its ligand, while outside-in signaling caused by ligand binding depends on FcαRI association with the Fc receptor gamma chain.
The following outline is provided as an overview of and topical guide to immunology:
Clark Lawrence Anderson is an internist and immunologist. He is professor emeritus in the Division of Immunology and Rheumatology, Department of Internal Medicine, Ohio State University (OSU), Columbus, Ohio, United States.
Elizabeth Sally Ward is a British physician who is Director of Translational Immunology at the Centre for Cancer Immunology in the University of Southampton. She was elected Fellow of the Royal Society in 2022.
References
- 1 2 Story CM, Mikulska JE, Simister NE (December 1994). "A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus". The Journal of Experimental Medicine. 180 (6): 2377–2381. doi:10.1084/jem.180.6.2377. PMC 2191771 . PMID 7964511.
- ↑ Kandil E, Egashira M, Miyoshi O, Niikawa N, Ishibashi T, Kasahara M, Miyosi O (July 1996). "The human gene encoding the heavy chain of the major histocompatibility complex class I-like Fc receptor (FCGRT) maps to 19q13.3". Cytogenetics and Cell Genetics. 73 (1–2): 97–98. doi:10.1159/000134316. PMID 8646894.
- ↑ "Entrez Gene: FCGRT Fc fragment of IgG, receptor, transporter, alpha".
- ↑ Simister NE, Mostov KE (1989). "Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog". Cold Spring Harbor Symposia on Quantitative Biology. 54 (Pt 1): 571–580. doi:10.1101/sqb.1989.054.01.068. PMID 2534798.
- ↑ Kuo TT, Aveson VG (2011-01-01). "Neonatal Fc receptor and IgG-based therapeutics". mAbs. 3 (5): 422–430. doi:10.4161/mabs.3.5.16983. PMC 3225846 . PMID 22048693.
- 1 2 3 Rodewald R, Kraehenbuhl JP (July 1984). "Receptor-mediated transport of IgG". The Journal of Cell Biology. 99 (1 Pt 2): 159s–164s. doi:10.1083/jcb.99.1.159s. PMC 2275593 . PMID 6235233.
- 1 2 3 Simister NE, Rees AR (July 1985). "Isolation and characterization of an Fc receptor from neonatal rat small intestine". European Journal of Immunology. 15 (7): 733–738. doi:10.1002/eji.1830150718. PMID 2988974. S2CID 42396197.
- 1 2 Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, et al. (August 2001). "The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans". International Immunology. 13 (8): 993–1002. doi: 10.1093/intimm/13.8.993 . PMID 11470769.
- 1 2 3 Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES (March 1996). "Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice". European Journal of Immunology. 26 (3): 690–696. doi:10.1002/eji.1830260327. PMID 8605939. S2CID 85730132.
- 1 2 3 Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL (February 2003). "The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan". The Journal of Experimental Medicine. 197 (3): 315–322. doi:10.1084/jem.20021829. PMC 2193842 . PMID 12566415.
- 1 2 Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, et al. (July 1997). "Increasing the serum persistence of an IgG fragment by random mutagenesis". Nature Biotechnology. 15 (7): 637–640. doi:10.1038/nbt0797-637. PMID 9219265. S2CID 39836528.
- 1 2 Roopenian DC, Akilesh S (September 2007). "FcRn: the neonatal Fc receptor comes of age". Nature Reviews. Immunology. 7 (9): 715–725. doi:10.1038/nri2155. PMID 17703228. S2CID 6980400.
- 1 2 Ward ES, Ober RJ (2009). Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Advances in Immunology. Vol. 103. pp. 77–115. doi:10.1016/S0065-2776(09)03004-1. PMC 4485553 . PMID 19755184.
- 1 2 Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, Blumberg RS (November 2010). "Neonatal Fc receptor: from immunity to therapeutics". Journal of Clinical Immunology. 30 (6): 777–789. doi:10.1007/s10875-010-9468-4. PMC 2970823 . PMID 20886282.
- ↑ Andersen JT, Dee Qian J, Sandlie I (November 2006). "The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin". European Journal of Immunology. 36 (11): 3044–3051. doi:10.1002/eji.200636556. PMID 17048273. S2CID 22024929.
- 1 2 3 Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, et al. (October 1999). "Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line". The Journal of Clinical Investigation. 104 (7): 903–911. doi:10.1172/JCI6968. PMC 408555 . PMID 10510331.
- ↑ Kim JK, Tsen MF, Ghetie V, Ward ES (October 1994). "Localization of the site of the murine IgG1 molecule that is involved in binding to the murine intestinal Fc receptor". European Journal of Immunology. 24 (10): 2429–2434. doi:10.1002/eji.1830241025. PMID 7925571. S2CID 43499403.
- ↑ Martin WL, West AP, Gan L, Bjorkman PJ (April 2001). "Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding". Molecular Cell. 7 (4): 867–877. doi: 10.1016/s1097-2765(01)00230-1 . PMID 11336709.
- ↑ Ward ES, Zhou J, Ghetie V, Ober RJ (February 2003). "Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans". International Immunology. 15 (2): 187–195. doi: 10.1093/intimm/dxg018 . PMID 12578848.
- ↑ Akilesh S, Christianson GJ, Roopenian DC, Shaw AS (October 2007). "Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism". Journal of Immunology. 179 (7): 4580–4588. doi: 10.4049/jimmunol.179.7.4580 . PMID 17878355.
- ↑ Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, et al. (July 2008). "Dependence of antibody-mediated presentation of antigen on FcRn". Proceedings of the National Academy of Sciences of the United States of America. 105 (27): 9337–9342. Bibcode:2008PNAS..105.9337Q. doi: 10.1073/pnas.0801717105 . PMC 2453734 . PMID 18599440.
- ↑ Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES (February 2009). "Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice". Proceedings of the National Academy of Sciences of the United States of America. 106 (8): 2788–2793. Bibcode:2009PNAS..106.2788M. doi: 10.1073/pnas.0810796106 . PMC 2650344 . PMID 19188594.
- 1 2 3 Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES (February 2004). "Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn". Journal of Immunology. 172 (4): 2021–2029. doi: 10.4049/jimmunol.172.4.2021 . PMID 14764666. S2CID 30526875.
- 1 2 Ober RJ, Martinez C, Lai X, Zhou J, Ward ES (July 2004). "Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level". Proceedings of the National Academy of Sciences of the United States of America. 101 (30): 11076–11081. Bibcode:2004PNAS..10111076O. doi: 10.1073/pnas.0402970101 . PMC 503743 . PMID 15258288.
- ↑ Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, et al. (April 2007). "Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy". Proceedings of the National Academy of Sciences of the United States of America. 104 (14): 5889–5894. doi: 10.1073/pnas.0700337104 . PMC 1851587 . PMID 17384151.
- ↑ Larsen MT, Rawsthorne H, Schelde KK, Dagnæs-Hansen F, Cameron J, Howard KA (October 2018). "Cellular recycling-driven in vivo half-life extension using recombinant albumin fusions tuned for neonatal Fc receptor (FcRn) engagement". Journal of Controlled Release. 287: 132–141. doi:10.1016/j.jconrel.2018.07.023. PMID 30016735. S2CID 51677989.
- ↑ Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI (August 2002). "Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung". The Journal of Experimental Medicine. 196 (3): 303–310. doi:10.1084/jem.20020400. PMC 2193935 . PMID 12163559.
- ↑ Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, et al. (January 2008). "Podocytes use FcRn to clear IgG from the glomerular basement membrane". Proceedings of the National Academy of Sciences of the United States of America. 105 (3): 967–972. doi: 10.1073/pnas.0711515105 . PMC 2242706 . PMID 18198272.
- ↑ Bern M, Sand KM, Nilsen J, Sandlie I, Andersen JT (August 2015). "The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery". Journal of Controlled Release. 211: 144–162. doi:10.1016/j.jconrel.2015.06.006. PMID 26055641. S2CID 205878058.
- ↑ Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT (2015-01-26). "Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics". Frontiers in Immunology. 5: 682. doi: 10.3389/fimmu.2014.00682 . PMC 4306297 . PMID 25674083.
- ↑ Pyzik M, Rath T, Kuo TT, Win S, Baker K, Hubbard JJ, et al. (April 2017). "Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury". Proceedings of the National Academy of Sciences of the United States of America. 114 (14): E2862–E2871. doi: 10.1073/pnas.1618291114 . PMC 5389309 . PMID 28330995.
- ↑ Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, et al. (2013-11-01). "The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells". PLOS Pathogens. 9 (11): e1003776. doi: 10.1371/journal.ppat.1003776 . PMC 3836734 . PMID 24278022.
- 1 2 Ward ES, Ober RJ (October 2018). "Targeting FcRn to Generate Antibody-Based Therapeutics". Trends in Pharmacological Sciences. 39 (10): 892–904. doi:10.1016/j.tips.2018.07.007. PMC 6169532 . PMID 30143244.
- ↑ "Ultomiris® (ravulizumab-cwvz) | Alexion" . Retrieved 2021-10-03.
- ↑ Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, et al. (November 2002). "Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences". Journal of Immunology. 169 (9): 5171–5180. doi: 10.4049/jimmunol.169.9.5171 . PMID 12391234. S2CID 29398244.
- ↑ "Coronavirus (COVID-19) Update: FDA Authorizes New Long-Acting Monoclonal Antibodies for Pre-exposure Prevention of COVID-19 in Certain Individuals". U.S. Food and Drug Administration. 8 December 2021.
- ↑ Andersen JT, Dalhus B, Viuff D, Ravn BT, Gunnarsen KS, Plumridge A, et al. (May 2014). "Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding". The Journal of Biological Chemistry. 289 (19): 13492–13502. doi: 10.1074/jbc.M114.549832 . PMC 4036356 . PMID 24652290.
- ↑ Lee TY, Tjin Tham Sjin RM, Movahedi S, Ahmed B, Pravda EA, Lo KM, et al. (March 2008). "Linking antibody Fc domain to endostatin significantly improves endostatin half-life and efficacy". Clinical Cancer Research. 14 (5): 1487–1493. doi: 10.1158/1078-0432.CCR-07-1530 . PMID 18316573.
- ↑ Poznansky MJ, Halford J, Taylor D (October 1988). "Growth hormone-albumin conjugates. Reduced renal toxicity and altered plasma clearance". FEBS Letters. 239 (1): 18–22. doi:10.1016/0014-5793(88)80537-4. PMID 3181423. S2CID 38592689.
- 1 2 3 Strohl WR (August 2015). "Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters". BioDrugs. 29 (4): 215–239. doi:10.1007/s40259-015-0133-6. PMC 4562006 . PMID 26177629.
- ↑ Goldenberg MM (January 1999). "Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis". Clinical Therapeutics. 21 (1): 75–87, discussion 1–2. doi: 10.1016/S0149-2918(00)88269-7 . PMID 10090426.
- ↑ Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D (May 2004). "The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease". The Journal of Clinical Investigation. 113 (9): 1328–1333. doi:10.1172/JCI18838. PMC 398424 . PMID 15124024.
- 1 2 Hansen RJ, Balthasar JP (June 2003). "Pharmacokinetic/pharmacodynamic modeling of the effects of intravenous immunoglobulin on the disposition of antiplatelet antibodies in a rat model of immune thrombocytopenia". Journal of Pharmaceutical Sciences. 92 (6): 1206–1215. doi:10.1002/jps.10364. PMID 12761810.
- 1 2 Patel DA, Puig-Canto A, Challa DK, Perez Montoyo H, Ober RJ, Ward ES (July 2011). "Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model". Journal of Immunology. 187 (2): 1015–1022. doi:10.4049/jimmunol.1003780. PMC 3157913 . PMID 21690327.
- ↑ Sockolosky JT, Szoka FC (August 2015). "The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy". Advanced Drug Delivery Reviews. Editor's Collection 2015. 91: 109–124. doi:10.1016/j.addr.2015.02.005. PMC 4544678 . PMID 25703189.
- ↑ Nimmerjahn F, Ravetch JV (2008-01-01). "Anti-inflammatory actions of intravenous immunoglobulin". Annual Review of Immunology. 26 (1): 513–533. doi:10.1146/annurev.immunol.26.021607.090232. PMID 18370923.
- 1 2 Vaccaro C, Zhou J, Ober RJ, Ward ES (October 2005). "Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels". Nature Biotechnology. 23 (10): 1283–1288. doi:10.1038/nbt1143. PMID 16186811. S2CID 13526188.
- ↑ Ulrichts P, Guglietta A, Dreier T, van Bragt T, Hanssens V, Hofman E, et al. (October 2018). "Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans". The Journal of Clinical Investigation. 128 (10): 4372–4386. doi:10.1172/JCI97911. PMC 6159959 . PMID 30040076.
- ↑ Nixon AE, Chen J, Sexton DJ, Muruganandam A, Bitonti AJ, Dumont J, et al. (2015). "Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates". Frontiers in Immunology. 6: 176. doi: 10.3389/fimmu.2015.00176 . PMC 4407741 . PMID 25954273.
- ↑ Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, et al. (November 2017). "The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study". Science Translational Medicine. 9 (414): eaan1208. doi: 10.1126/scitranslmed.aan1208 . PMID 29093180. S2CID 206694327.
- ↑ Blumberg LJ, Humphries JE, Jones SD, Pearce LB, Holgate R, Hearn A, et al. (December 2019). "Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses". Science Advances. 5 (12): eaax9586. Bibcode:2019SciA....5.9586B. doi:10.1126/sciadv.aax9586. PMC 6920022 . PMID 31897428.
- ↑ Newland AC, Sánchez-González B, Rejtő L, Egyed M, Romanyuk N, Godar M, et al. (February 2020). "Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia". American Journal of Hematology. 95 (2): 178–187. doi:10.1002/ajh.25680. PMC 7004056 . PMID 31821591.
- ↑ Robak T, Kaźmierczak M, Jarque I, Musteata V, Treliński J, Cooper N, et al. (September 2020). "Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia". Blood Advances. 4 (17): 4136–4146. doi:10.1182/bloodadvances.2020002003. PMC 7479959 . PMID 32886753.
- ↑ Werth VP, Culton DA, Concha JS, Graydon JS, Blumberg LJ, Okawa J, et al. (December 2021). "Safety, Tolerability, and Activity of ALXN1830 Targeting the Neonatal Fc Receptor in Chronic Pemphigus". The Journal of Investigative Dermatology. 141 (12): 2858–2865.e4. doi: 10.1016/j.jid.2021.04.031 . PMID 34126109. S2CID 235439165.
- ↑ Goebeler M, Bata-Csörgő Z, De Simone C, Didona B, Remenyik E, Reznichenko N, et al. (October 2021). "Treatment of pemphigus vulgaris and foliaceus with efgartigimod, a neonatal Fc receptor inhibitor: a phase II multicentre, open-label feasibility trial". The British Journal of Dermatology. 186 (3): 429–439. doi: 10.1111/bjd.20782 . PMID 34608631. S2CID 238355823.
- ↑ "argenx Announces U.S. Food and Drug Administration (FDA) Approval of VYVGART™ (efgartigimod alfa-fcab) in Generalized Myasthenia Gravis". Argenx. 17 December 2021.