Everglades National Park is an American national park that protects the southern twenty percent of the original Everglades in Florida. The park is the largest tropical wilderness in the United States and the largest wilderness of any kind east of the Mississippi River. An average of one million people visit the park each year. Everglades is the third-largest national park in the contiguous United States after Death Valley and Yellowstone. UNESCO declared the Everglades & Dry Tortugas Biosphere Reserve in 1976 and listed the park as a World Heritage Site in 1979, and the Ramsar Convention included the park on its list of Wetlands of International Importance in 1987. Everglades is one of only three locations in the world to appear on all three lists.

The Key deer is an endangered subspecies of the white-tailed deer that lives only in the Florida Keys. It is the smallest extant North American deer species.

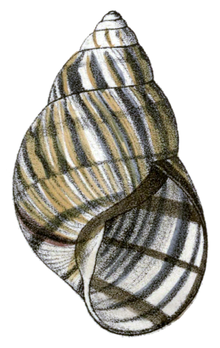
Achatinella is a tropical genus of colorful land snails in the monotypic Achatinellidae subfamily Achatinellinae.

The Key Largo woodrat, a subspecies of the eastern woodrat, is a medium-sized rat found on less than 2,000 acres of the northern area of Key Largo, Florida, in the United States. It is currently on the United States Fish and Wildlife Service list of endangered species. Only 6500 animals were thought to remain in North Key Largo in the late 1980s.

Hammock is a term used in the southeastern United States for stands of trees, usually hardwood, that form an ecological island in a contrasting ecosystem. Hammocks grow on elevated areas, often just a few inches high, surrounded by wetlands that are too wet to support them. The term hammock is also applied to stands of hardwood trees growing on slopes between wetlands and drier uplands supporting a mixed or coniferous forest. Types of hammocks found in the United States include tropical hardwood hammocks, temperate hardwood hammocks, and maritime or coastal hammocks. Hammocks are also often classified as hydric, mesic or xeric. The types are not exclusive, but often grade into each other.
The Crocodile Lake National Wildlife Refuge is part of the United States National Wildlife Refuge System, located in north Key Largo, less than 40 miles (64 km) south of Miami off SR 905. The 6,686 acre (27.1 km2) refuge opened during the year of 1980, under the Land and Water Conservation Fund Act of 1965 and the Endangered Species Act of 1973. It was established in order to protect critical breeding and nesting habitat for the threatened American crocodile and other wildlife. This area also includes 650 acres (2.6 km2) of open water in and around the refuge. In addition to being one of only three breeding populations of the American crocodile, the refuge is home to tropical hardwood hammock, mangrove forest, and salt marsh. It is administered as part of the National Key Deer Refuge which is also located in the Florida Keys.

The Miami blue is a small butterfly that is native to coastal areas of southern Florida. It is a subspecies of Thomas's blue. Once common throughout its range, it has become critically endangered, and is considered to be near extinction. Its numbers have recently been increased by a captive breeding program at the Florida Museum of Natural History.

The Kanab ambersnail, formerly classified as Oxyloma haydeni kanabense or Oxyloma kanabense, is a small, air-breathing land snail belonging to the family Succineidae, the ambersnails. This terrestrial pulmonate gastropod mollusc was previously considered a critically endangered subspecies or species. In 2013, a scientific investigations report by the United States Geological Survey concluded that the Kanab ambersnail is not a genetically distinct species. In June 2021, the Fish and Wildlife Service removed the Kanab ambersnail from the United States Fish and Wildlife Service list of endangered mammals and birds and classified it with other common ambersnails within the same taxa, officially negating its status as a distinct subspecies.

Newcombia cumingi, common name Newcomb's Tree snail, is a species of air-breathing land snail, a terrestrial pulmonate gastropod mollusk in the family Achatinellidae. This species is endemic to Hawaii, the United States.

Tree snail is a common name that is applied to various kinds of tropical air-breathing land snails, pulmonate gastropod mollusks that have shells, and that live in trees, in other words, are exclusively arboreal in habitat.

The Chittenango ovate amber snail is a species of small air-breathing land snail in the family Succineidae, the amber snails. This species was discovered in 1905, and was reported three years later as a subspecies of the oval ambersnail, Succinea ovalis. Several taxonomic reviews took place in the subsequent decades until the end of the 1980s, when the Chittenango ovate amber snail was finally judged to be a distinct species based on chemical and morphological data.
The flat-spired three-toothed snail —also known as the Cheat three-toothed snail after the Cheat River in West Virginia—is a species of air-breathing land snail, a terrestrial pulmonate gastropod mollusk in the family Polygyridae.

Liguus is a genus of large tropical air-breathing land snails, more specifically arboreal or tree snails, terrestrial pulmonate gastropod mollusks in the family Orthalicidae.

Sylvilagus palustris hefneri, also known commonly as the Lower Keys marsh rabbit, is an endangered subspecies of marsh rabbit in the family Leporidae. The subspecies is named after Playboy founder Hugh Hefner.

The Socorro springsnail, scientific name Pyrgulopsis neomexicana, is an endangered species of minute freshwater snail with a gill and an operculum, an aquatic gastropod mollusk or micromollusk in the family Hydrobiidae, the mud snails.

Liguus fasciatus is a species of air-breathing land snail, a tree snail, a terrestrial pulmonate gastropod mollusk in the family Orthalicidae.

Orthalicus is a genus of land snails in the family Orthalicidae.

Papilio aristodemus, the Schaus' swallowtail or island swallowtail, is a species of American butterfly in the family Papilionidae. It is found in southern Florida in the United States and throughout the West Indies. It is named in honor of William Schaus.

Tropical hardwood hammocks are closed canopy forests, dominated by a diverse assemblage of evergreen and semi-deciduous tree and shrub species, mostly of West Indian origin. Tropical hardwood hammocks are found in South Florida or the Everglades, with large concentrations on the Miami Rock Ridge, in the Florida Keys, along the northern shores of Florida Bay, and in the Pinecrest region of the Big Cypress Swamp.
The Key Largo cotton mouse is a subspecies of rodent in the family Cricetidae. The subspecies is endemic to Key Largo in the upper Florida Keys. It is a slightly larger mouse with a more reddish color than other mouse species from mainland Florida. The Key Largo cotton mouse can breed throughout the year and has an average life expectancy of five months.