Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word crystallography is derived from the Ancient Greek word κρύσταλλος, and γράφειν. In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming 2014 the International Year of Crystallography.

X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information.

A chemical structure of a molecule is a spatial arrangement of its atoms and their chemical bonds. Its determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together and can be represented using structural formulae and by molecular models; complete electronic structure descriptions include specifying the occupation of a molecule's molecular orbitals. Structure determination can be applied to a range of targets from very simple molecules to very complex ones.

Henry Taube, was a Canadian-born American chemist who was awarded the 1983 Nobel Prize in Chemistry for "his work in the mechanisms of electron-transfer reactions, especially in metal complexes." He was the second Canadian-born chemist to win the Nobel Prize, and remains the only Saskatchewanian-born Nobel laureate. Taube completed his undergraduate and master's degrees at the University of Saskatchewan, and his PhD from the University of California, Berkeley. After finishing graduate school, Taube worked at Cornell University, the University of Chicago and Stanford University.
The Ewald sphere is a geometric construction used in electron, neutron, and x-ray diffraction which shows the relationship between:

Herbert Aaron Hauptman was an American mathematician and Nobel laureate. He pioneered and developed a mathematical method that has changed the whole field of chemistry and opened a new era in research in determination of molecular structures of crystallized materials. Today, Hauptman's direct methods, which he continued to improve and refine, are routinely used to solve complicated structures. It was the application of this mathematical method to a wide variety of chemical structures that led the Royal Swedish Academy of Sciences to name Hauptman and Jerome Karle recipients of the 1985 Nobel Prize in Chemistry.

Sir John Meurig Thomas, also known as JMT, was a Welsh scientist, educator, university administrator, and historian of science primarily known for his work on heterogeneous catalysis, solid-state chemistry, and surface and materials science.
Michael G. Rossmann was a German-American physicist, microbiologist, and Hanley Distinguished Professor of Biological Sciences at Purdue University who led a team of researchers to be the first to map the structure of a human common cold virus to an atomic level. He also discovered the Rossmann fold protein motif. His most well recognised contribution to structural biology is the development of a phasing technique named molecular replacement, which has led to about three quarters of depositions in the Protein Data Bank.

Michael Stanley Whittingham is a British-American chemist. He is a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, State University of New York. He also serves as director of the Northeastern Center for Chemical Energy Storage (NECCES) of the U.S. Department of Energy at Binghamton. He was awarded the Nobel Prize in Chemistry in 2019 alongside Akira Yoshino and John B. Goodenough.

This timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of matter and of its interactions.
The International Union of Crystallography (IUCr) is an organisation devoted to the international promotion and coordination of the science of crystallography. The IUCr is a member of the International Council for Science (ICSU).
Resolution in the context of structural biology is the ability to distinguish the presence or absence of atoms or groups of atoms in a biomolecular structure. Usually, the structure originates from methods such as X-ray crystallography, electron crystallography, or cryo-electron microscopy. The resolution is measured of the "map" of the structure produced from experiment, where an atomic model would then be fit into. Due to their different natures and interactions with matter, in X-ray methods the map produced is of the electron density of the system, whereas in electron methods the map is of the electrostatic potential of the system. In both cases, atomic positions are assumed similarly.
Nuclear magnetic resonance crystallography is a method which utilizes primarily NMR spectroscopy to determine the structure of solid materials on the atomic scale. Thus, solid-state NMR spectroscopy would be used primarily, possibly supplemented by quantum chemistry calculations, powder diffraction etc. If suitable crystals can be grown, any crystallographic method would generally be preferred to determine the crystal structure comprising in case of organic compounds the molecular structures and molecular packing. The main interest in NMR crystallography is in microcrystalline materials which are amenable to this method but not to X-ray, neutron and electron diffraction. This is largely because interactions of comparably short range are measured in NMR crystallography.
David Sayre was an American scientist, credited with the early development of direct methods for protein crystallography and of diffraction microscopy. While working at IBM he was part of the initial team of ten programmers who created FORTRAN, and later suggested the use of electron beam lithography for the fabrication of X-ray Fresnel zone plates.

John Charles Howorth Spence ForMemRS HonFRMS was Richard Snell Professor of Physics at Arizona State University and Director of Science at the National Science Foundation BioXFEL Science and Technology Center.
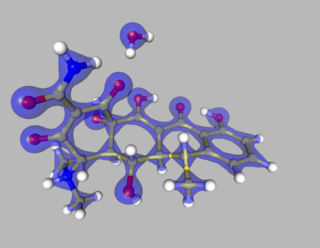
The Multipole Density Formalism is an X-ray crystallography method of electron density modelling proposed by Niels K. Hansen and Philip Coppens in 1978. Unlike the commonly used Independent Atom Model, the Hansen-Coppens Formalism presents an aspherical approach, allowing one to model the electron distribution around a nucleus separately in different directions and therefore describe numerous chemical features of a molecule inside the unit cell of an examined crystal in detail.
Quantum crystallography is a branch of crystallography that investigates crystalline materials within the framework of quantum mechanics, with analysis and representation, in position or in momentum space, of quantities like wave function, electron charge and spin density, density matrices and all properties related to them. Like the quantum chemistry, Quantum crystallography involves both experimental and computational work. The theoretical part of quantum crystallography is based on quantum mechanical calculations of atomic/molecular/crystal wave functions, density matrices or density models, used to simulate the electronic structure of a crystalline material. While in quantum chemistry, the experimental works mainly rely on spectroscopy, in quantum crystallography the scattering techniques play the central role, although spectroscopy as well as atomic microscopy are also sources of information.
In 1986 the International Union of Crystallography (IUCr) established the Ewald Prize for outstanding contributions to the science of crystallography. The Ewald Prize is considered the highest prize available to crystallographers apart from the Nobel Prize. The Ewald Prize has been described as prestigious, acclaimed and coveted.
This is a timeline of crystallography.
Boris Konstantinovich Vainshtein was a Russian crystallographer. He headed the Laboratory of Protein Crystallography of the Shubnikov Institute of Crystallography RAS, and was the director of the institute, where he spent the majority of his career.