Related Research Articles
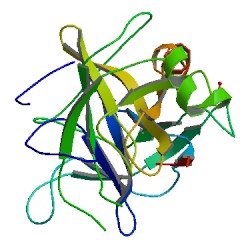
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine and methionine at the P1 position.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion.

A protease is an enzyme that catalyzes proteolysis, the breakdown of proteins into smaller polypeptides or single amino acids. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism, and cell signaling.

Thyrotropin-releasing hormone (TRH) is a hypophysiotropic hormone produced by neurons in the hypothalamus that stimulates the release of thyroid-stimulating hormone (TSH) and prolactin from the anterior pituitary.
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to another carboxylic group in a protein to make it a chain, but since the end amino acid of a protein is only connected at the carboxy- end, the remaining free amine group is called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction (because when a protein is translated from messenger RNA, it is created from N-terminus to C-terminus - amino acids are added to the carboxyl end).
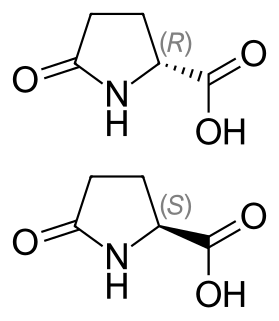
Pyroglutamic acid is a ubiquitous but little studied natural amino acid derivative in which the free amino group of glutamic acid or glutamine cyclizes to form a lactam. The names of pyroglutamic acid conjugate base, anion, salts, and esters are pyroglutamate, 5-oxoprolinate, or pidolate.
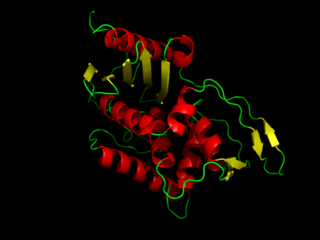
DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.
An exopeptidase is any peptidase that catalyzes the cleavage of the terminal peptide bond; the process releases a single amino acid or dipeptide from the peptide chain. Depending on whether the amino acid is released from the amino or the carboxy terminal, an exopeptidase is further classified as an aminopeptidase or a carboxypeptidase, respectively. Thus, an aminopeptidase, an enzyme in the brush border of the small intestine, will cleave a single amino acid from the amino terminal, whereas carboxypeptidase, which is a digestive enzyme present in pancreatic juice, will cleave a single amino acid from the carboxylic end of the peptide.
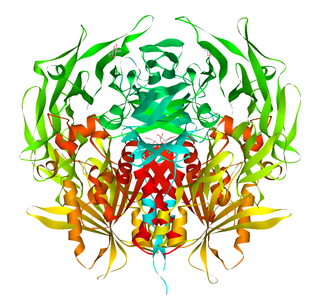
Dipeptidyl peptidase-4 (DPP4), also known as adenosine deaminase complexing protein 2 or CD26 is a protein that, in humans, is encoded by the DPP4 gene. DPP4 is related to FAP, DPP8, and DPP9. The enzyme was discovered in 1966 by Hopsu-Havu and Glenner, and as a result of various studies on chemism, was called dipeptidyl peptidase IV [DP IV].
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides (exopeptidases).They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes.
Protein metabolism denotes the various biochemical processes responsible for the synthesis of proteins and amino acids (anabolism), and the breakdown of proteins by catabolism.
Glutamyl aminopeptidase (EC 3.4.11.7, aminopeptidase A, aspartate aminopeptidase, angiotensinase A, glutamyl peptidase, Ca2+-activated glutamate aminopeptidase, membrane aminopeptidase II, antigen BP-1/6C3 of mouse B lymphocytes, L-aspartate aminopeptidase, angiotensinase A2) is an enzyme encoded by the ENPEP gene. Glutamyl aminopeptidase has also recently been designated CD249 (cluster of differentiation 249).

Chromosome 9 open reading frame 3 (C9ORF3) also known as aminopeptidase O (APO) is an enzyme which in humans is encoded by the C9ORF3 gene. The protein encoded by this gene is an aminopeptidase which is most closely related in sequence to leukotriene A4 hydrolase (LTA4H). APO is a member of the M1 metalloproteinase family.
Methionyl aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
PepB aminopeptidase is an enzyme which catalyses the following chemical reaction:
Acylaminoacyl-peptidase is an enzyme. This enzyme catalyses the following chemical reaction
Pyroglutamyl-peptidase I (EC 3.4.19.3, also known as Pyrrolidonyl peptidase, is an enzyme found in bacteria, plants and animals.
Pyroglutamyl-peptidase II is an enzyme. This enzyme catalyses the following chemical reaction
An endopeptidase inhibitor is a drug that inhibits one or more endopeptidase enzymes. Endopeptidases are one of two types of proteases, the other being exopeptidases. Endopeptidases cleave peptide bonds of non-terminal amino acids, whereas exopeptidases break terminal bonds, resulting in the release of a single amino acid or dipeptide from the peptide chain.
References
- ↑ Mozdzanowski, Jacek; Bongers, Jacob; Anumula, Kalyan (1 July 1998). "High-Yield Deblocking of Amino Termini of Recombinant Immunoglobulins with Pyroglutamate Aminopeptidase". Analytical Biochemistry. 260 (2): 183–187. doi:10.1006/abio.1998.2690. PMID 9657876.