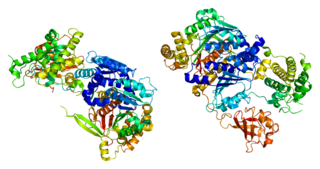Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ubiquitously. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A.

A ubiquitin ligase is a protein that recruits an E2 ubiquitin-conjugating enzyme that has been loaded with ubiquitin, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another protein by some mechanism. The ubiquitin, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases regulates diverse areas such as cell trafficking, DNA repair, and signaling and is of profound importance in cell biology. E3 ligases are also key players in cell cycle control, mediating the degradation of cyclins, as well as cyclin dependent kinase inhibitor proteins. The human genome encodes over 600 putative E3 ligases, allowing for tremendous diversity in substrates.
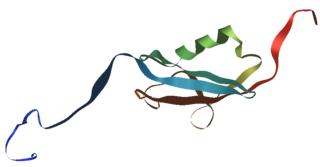
In molecular biology, SUMOproteins are a family of small proteins that are covalently attached to and detached from other proteins in cells to modify their function. This process is called SUMOylation. SUMOylation is a post-translational modification involved in various cellular processes, such as nuclear-cytosolic transport, transcriptional regulation, apoptosis, protein stability, response to stress, and progression through the cell cycle.
Abgent is a global biotechnology company based in San Diego, California, US with offices in Maidenhead, UK and Suzhou, China and distributors around the world. Abgent develops antibodies and related agents to study proteins involved in cellular function and disease. Abgent's antibodies target key areas of research including autophagy, neuroscience, cancer, stem cells and more. Abgent was acquired in 2011 by WuXi AppTec, a global pharmaceutical, biopharmaceutical, and medical device outsourcing company with operations in China and the United States.

Histone-modifying enzymes are enzymes involved in the modification of histone substrates after protein translation and affect cellular processes including gene expression. To safely store the eukaryotic genome, DNA is wrapped around four core histone proteins, which then join to form nucleosomes. These nucleosomes further fold together into highly condensed chromatin, which renders the organism's genetic material far less accessible to the factors required for gene transcription, DNA replication, recombination and repair. Subsequently, eukaryotic organisms have developed intricate mechanisms to overcome this repressive barrier imposed by the chromatin through histone modification, a type of post-translational modification which typically involves covalently attaching certain groups to histone residues. Once added to the histone, these groups elicit either a loose and open histone conformation, euchromatin, or a tight and closed histone conformation, heterochromatin. Euchromatin marks active transcription and gene expression, as the light packing of histones in this way allows entry for proteins involved in the transcription process. As such, the tightly packed heterochromatin marks the absence of current gene expression.

Small ubiquitin-related modifier 1 is a protein that in humans is encoded by the SUMO1 gene.

SUMO-conjugating enzyme UBC9 is an enzyme that in humans is encoded by the UBE2I gene. It is also sometimes referred to as "ubiquitin conjugating enzyme E2I" or "ubiquitin carrier protein 9", even though these names do not accurately describe its function.

E3 SUMO-protein ligase PIAS1 is an enzyme that in humans is encoded by the PIAS1 gene.

Ran GTPase-activating protein 1 is an enzyme that in humans is encoded by the RANGAP1 gene.

Small ubiquitin-related modifier 3 is a protein that in humans is encoded by the SUMO3 gene.
SUMO-activating enzyme subunit 1 is a protein that in humans is encoded by the SAE1 gene.

Ubiquitin-conjugating enzyme E2 G2 is a protein that in humans is encoded by the UBE2G2 gene.

NEDD8-activating enzyme E1 catalytic subunit is a protein that in humans is encoded by the UBA3 gene.

Sentrin-specific protease 2 is an enzyme that in humans is encoded by the SENP2 gene.
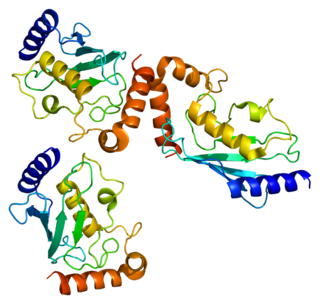
Ubiquitin-conjugating enzyme E2 E1 is a protein that in humans is encoded by the UBE2E1 gene.

NEDD8-conjugating enzyme Ubc12 is a protein that in humans is encoded by the UBE2M gene.
Ubiquitin-like 1-activating enzyme E1B (UBLE1B) also known as SUMO-activating enzyme subunit 2 (SAE2) is an enzyme that in humans is encoded by the UBA2 gene.
Ulp1 peptidase is an enzyme. This enzyme catalyses the following chemical reaction

Ubiquitin-like proteins (UBLs) are a family of small proteins involved in post-translational modification of other proteins in a cell, usually with a regulatory function. The UBL protein family derives its name from the first member of the class to be discovered, ubiquitin (Ub), best known for its role in regulating protein degradation through covalent modification of other proteins. Following the discovery of ubiquitin, many additional evolutionarily related members of the group were described, involving parallel regulatory processes and similar chemistry. UBLs are involved in a widely varying array of cellular functions including autophagy, protein trafficking, inflammation and immune responses, transcription, DNA repair, RNA splicing, and cellular differentiation.
Arabidopsis SUMO-conjugation enzyme (AtSCE1) is an enzyme that is a member of the small ubiquitin-like modifier (SUMO) post-translational modification pathway. This process, and the SCE1 enzyme with it, is highly conserved across eukaryotes yet absent in prokaryotes. In short, this pathway results in the attachment of a small polypeptide through an isopeptide bond between modifying enzyme and the ε-amino group of a lysine residue in the substrate. In plants, the 160 amino acid SCE1 enzyme was first characterized in 2003. One functional gene copy, SCE1a, was found on chromosomes 3.