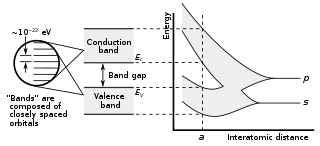
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from the valence band to the conduction band, then current can flow. Therefore, the band gap is a major factor determining the electrical conductivity of a solid. Substances with large band gaps are generally insulators, those with smaller band gaps are semiconductors, while conductors either have very small band gaps or none, because the valence and conduction bands overlap to form a continuous band.

A laser diode is a semiconductor device similar to a light-emitting diode in which a diode pumped directly with electrical current can create lasing conditions at the diode's junction.

Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.
Wide-bandgap semiconductors are semiconductor materials which have a larger band gap than conventional semiconductors. Conventional semiconductors like silicon have a bandgap in the range of 0.6 – 1.5 electronvolt (eV), whereas wide-bandgap materials have bandgaps in the range above 2 eV. Generally, wide-bandgap semiconductors have electronic properties which fall in between those of conventional semiconductors and insulators.

Indium phosphide (InP) is a binary semiconductor composed of indium and phosphorus. It has a face-centered cubic ("zincblende") crystal structure, identical to that of GaAs and most of the III-V semiconductors.

A quantum well is a potential well with only discrete energy values.

Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors.
An atomic battery, nuclear battery, radioisotope battery or radioisotope generator is a device which uses energy from the decay of a radioactive isotope to generate electricity. Like nuclear reactors, they generate electricity from nuclear energy, but differ in that they do not use a chain reaction. Although commonly called batteries, they are technically not electrochemical and cannot be charged or recharged. They are very costly, but have an extremely long life and high energy density, and so they are typically used as power sources for equipment that must operate unattended for long periods of time, such as spacecraft, pacemakers, underwater systems and automated scientific stations in remote parts of the world.

A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon. It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as current, voltage, or resistance, vary when exposed to light. Individual solar cell devices are often the electrical building blocks of photovoltaic modules, known colloquially as solar panels. The common single junction silicon solar cell can produce a maximum open-circuit voltage of approximately 0.5 volts to 0.6volts.

A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the absorbing photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are tunable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum.
Thermophotonics is a concept for generating usable power from heat which shares some features of thermophotovoltaic (TPV) power generation. Thermophotonics was first publicly proposed by solar photovoltaic researcher Martin Green in 2000. However, no TPX device is known to have been demonstrated to date, apparently because of the stringent requirement on the emitter efficiency.

Multi-junction (MJ) solar cells are solar cells with multiple p–n junctions made of different semiconductor materials. Each material's p-n junction will produce electric current in response to different wavelengths of light. The use of multiple semiconducting materials allows the absorbance of a broader range of wavelengths, improving the cell's sunlight to electrical energy conversion efficiency.

In physics, the radiative efficiency limit is the maximum theoretical efficiency of a solar cell using a single p-n junction to collect power from the cell where the only loss mechanism is radiative recombination in the solar cell. It was first calculated by William Shockley and Hans-Joachim Queisser at Shockley Semiconductor in 1961, giving a maximum efficiency of 30% at 1.1 eV. The limit is one of the most fundamental to solar energy production with photovoltaic cells, and is considered to be one of the most important contributions in the field.
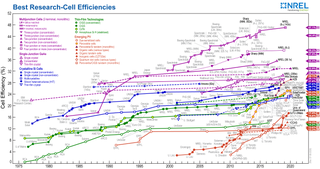
There are currently many research groups active in the field of photovoltaics in universities and research institutions around the world. This research can be categorized into three areas: making current technology solar cells cheaper and/or more efficient to effectively compete with other energy sources; developing new technologies based on new solar cell architectural designs; and developing new materials to serve as more efficient energy converters from light energy into electric current or light absorbers and charge carriers.
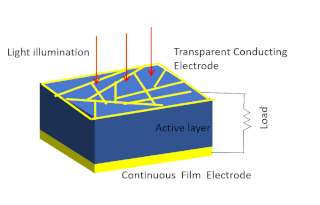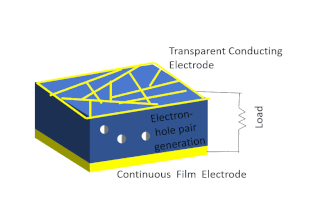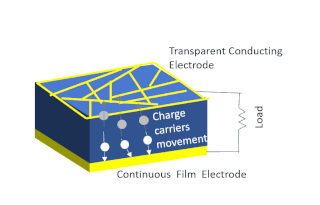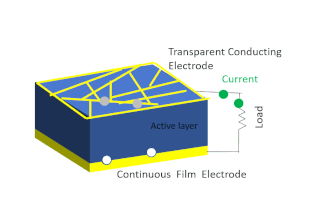
Solar-cell efficiency refers to the portion of energy in the form of sunlight that can be converted via photovoltaics into electricity by the solar cell.
Thermodynamic efficiency limit is the absolute maximum theoretically possible conversion efficiency of sunlight to electricity. Its value is about 86%, which is the Chambadal-Novikov efficiency, an approximation related to the Carnot limit, based on the temperature of the photons emitted by the Sun's surface.
Sun-free photovoltaics is a photovoltaics technology which does not require sunlight to produce electricity. This technique was developed by research team at Massachusetts Institute of Technology. Photovoltaic cells convert light to electricity most efficiently at specific wavelengths. The surface features of Sun-free photovoltaics is engineered such that it converts heat energy into the specific wavelengths. This increases the efficiency of existing thermophotovoltaic (TPV) systems.
Two-photon photovoltaic effect is an energy collection method based on two-photon absorption (TPA). The TPP effect can be thought of as the nonlinear equivalent of the traditional photovoltaic effect involving high optical intensities. This effect occurs when two photons are absorbed at the same time resulting in an electron-hole pair.
Solar energy – radiant light and heat from the sun. It has been harnessed by humans since ancient times using a range of ever-evolving technologies. Solar energy technologies include solar heating, solar photovoltaics, solar thermal electricity and solar architecture, which can make considerable contributions to solving some of the most urgent problems that the world now faces.
Light-emitting diodes (LEDs) produce light by the recombination of electrons and electron holes in a semiconductor, a process called "electroluminescence". The wavelength of the light produced depends on the energy band gap of the semiconductors used. Since these materials have a high index of refraction, design features of the devices such as special optical coatings and die shape are required to efficiently emit light. A LED is a long-lived light source, but certain mechanisms can cause slow loss of efficiency of the device or sudden failure. The wavelength of the light emitted is a function of the band gap of the semiconductor material used; materials such as gallium arsenide, and others, with various trace doping elements, are used to produce different colors of light. Another type of LED uses a quantum dot which can have its properties and wavelength adjusted by its size. Light-emitting diodes are widely used in indicator and display functions, and white LEDs are displacing other technologies for general illumination purposes.















