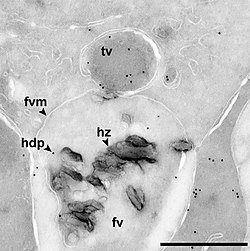
In cell biology, a vesicle is an organelle within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes (not to be confused with lysosomes). If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes ; otherwise they are called multilamellar liposomes. [1] The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.
Contents
- Types of vesicular structures
- Vacuoles
- Lysosomes
- Transport vesicles
- Secretory vesicles
- Extracellular vesicles
- Protocells
- Other types
- Formation and transport
- Vesicle coat and cargo molecules
- Vesicle docking
- Vesicle fusion
- In receptor downregulation
- Preparation
- Artificial vesicles
- See also
- References
- Further reading
- External links
Vesicles perform a variety of functions. Because it is separated from the cytosol, the inside of the vesicle can be made to be different from the cytosolic environment. For this reason, vesicles are a basic tool used by the cell for organizing cellular substances. Vesicles are involved in metabolism, transport, buoyancy control, [2] and temporary storage of food and enzymes. They can also act as chemical reaction chambers.
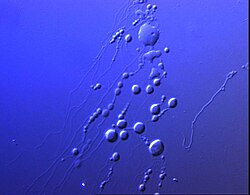
Closed structure formed by amphiphilic molecules that contains solvent (usually water). [3]
The 2013 Nobel Prize in Physiology or Medicine was shared by James Rothman, Randy Schekman and Thomas Südhof for their roles in elucidating (building upon earlier research, some of it by their mentors) the makeup and function of cell vesicles, especially in yeasts and in humans, including information on each vesicle's parts and how they are assembled. Vesicle dysfunction is thought to contribute to Alzheimer's disease, diabetes, some hard-to-treat cases of epilepsy, some cancers and immunological disorders and certain neurovascular conditions. [4] [5]