
A contractile vacuole (CV) is a sub-cellular structure (organelle) involved in osmoregulation. It is found predominantly in protists, including unicellular algae. It was previously known as pulsatile or pulsating vacuole.

A contractile vacuole (CV) is a sub-cellular structure (organelle) involved in osmoregulation. It is found predominantly in protists, including unicellular algae. It was previously known as pulsatile or pulsating vacuole.
The contractile vacuole is a specialized type of vacuole that regulates the quantity of water inside a cell. In freshwater environments, the concentration of solutes is hypotonic, lower outside than inside the cell. Under these conditions, osmosis causes water to accumulate in the cell from the external environment. The contractile vacuole acts as part of a protective mechanism that prevents the cell from absorbing too much water and possibly lysing (rupturing) through excessive internal pressure.
The contractile vacuole, as its name suggests, expels water out of the cell by contracting. The growth (water gathering) and contraction (water expulsion) of the contractile vacuole are periodical. One cycle takes several seconds, depending on the species and the osmolarity of the environment. The stage in which water flows into the CV is called diastole. The contraction of the contractile vacuole and the expulsion of water out of the cell is called systole.
Water always flows first from outside the cell into the cytoplasm, and is only then moved from the cytoplasm into the contractile vacuole for expulsion. Species that possess a contractile vacuole typically always use the organelle, even at very hypertonic (high concentration of solutes) environments, since the cell tends to adjust its cytoplasm to become even more hyperosmotic than the environment. The amount of water expelled from the cell and the rate of contraction are related to the osmolarity of the environment. In hyperosmotic environments, less water will be expelled and the contraction cycle will be longer.
The best-understood contractile vacuoles belong to the protists Paramecium , Amoeba , Dictyostelium and Trypanosoma , and to a lesser extent the green alga Chlamydomonas . Not all species that possess a contractile vacuole are freshwater organisms; some marine, soil microorganisms and parasites also have a contractile vacuole. The contractile vacuole is predominant in species that do not have a cell wall, but there are exceptions (notably Chlamydomonas) which do possess a cell wall. Through evolution, the contractile vacuole has typically been lost in multicellular organisms, but it still exists in the unicellular stage of several multicellular fungi, as well as in several types of cells in sponges (amoebocytes, pinacocytes, and choanocytes). [1]
The number of contractile vacuoles per cell varies, depending on the species. Amoeba have one, Dictyostelium discoideum , Paramecium aurelia and Chlamydomonas reinhardtii have two, and giant amoeba, such as Chaos carolinensis , have many. The number of contractile vacuoles in each species is mostly constant and is therefore used for species characterization in systematics. The contractile vacuole has several structures attached to it in most cells, such as membrane folds, tubules, water tracts and small vesicles. These structures have been termed the spongiome; the contractile vacuole together with the spongiome is sometimes called the "contractile vacuole complex" (CVC). The spongiome serves several functions in water transport into the contractile vacuole and in localization and docking of the contractile vacuole within the cell.
Paramecium and Amoeba possess large contractile vacuoles (average diameter of 13 and 45 μm, respectively), which are relatively comfortable to isolate, manipulate and assay. The smallest known contractile vacuoles belong to Chlamydomonas, with a diameter of 1.5 μm. In Paramecium, which has one of the most complex contractile vacuoles, the vacuole is surrounded by several canals, which absorb water by osmosis from the cytoplasm. After the canals fill with water, the water is pumped into the vacuole. When the vacuole is full, it expels the water through a pore in the cytoplasm which can be opened and closed. [2] Other protists, such as Amoeba , have CVs that move to the surface of the cell when full and undergo exocytosis. In Amoeba contractile vacuoles collect excretory waste, such as ammonia, from the intracellular fluid by both diffusion and active transport.

The way in which water enters the CV had been a mystery for many years, but several discoveries since the 1990s have improved understanding of this issue. Water could theoretically cross the CV membrane by osmosis, but only if the inside of the CV is hyperosmotic (higher solute concentration) to the cytoplasm. The discovery of proton pumps in the CV membrane [3] and the direct measurement of ion concentrations inside the CV using microelectrodes [4] led to the following model: the pumping of protons either into or out of the CV causes different ions to enter the CV. For example, some proton pumps work as cation exchangers, whereby a proton is pumped out of the CV and a cation is pumped at the same time into the CV. In other cases, protons pumped into the CV drag anions with them (carbonate, for example), to balance the pH. This ion flux into the CV causes an increase in CV osmolarity and as a result water enters the CV by osmosis. Water has been shown in at least some species to enter the CV through aquaporins. [5]
Acidocalcisomes have been implied to work alongside the contractile vacuole in responding to osmotic stress. They were detected in the vicinity of the vacuole in Trypanosoma cruzi and were shown to fuse with the vacuole when the cells were exposed to osmotic stress. Presumably the acidocalcisomes empty their ion contents into the contractile vacuole, thereby increasing the vacuole's osmolarity. [6]
This section possibly contains original research .(July 2010) |
The CV does not exist in higher organisms, but some of its unique characteristics are used by them in their osmoregulatory mechanisms. Research on the CV can therefore help us understand how osmoregulation works in all species. Many issues regarding the CV remain, as of 2010, unsolved:
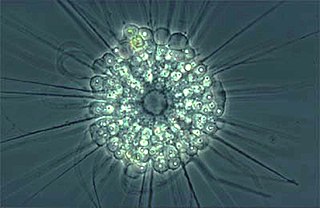
The actinophryids are an order of heliozoa, a polyphyletic array of stramenopiles, having a close relationship with pedinellids and Ciliophrys. They are common in fresh water and occasionally found in marine and soil habitats. Actinophryids are unicellular and roughly spherical in shape, with many axopodia that radiate outward from the cell body. Axopodia are a type of pseudopodia that are supported by hundreds of microtubules arranged in interlocking spirals and forming a needle-like internal structure or axoneme. Small granules, extrusomes, that lie under the membrane of the body and axopodia capture flagellates, ciliates and small metazoa that make contact with the arms.

The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol, the cell's internal sub-structures, and various cytoplasmic inclusions. In eukaryotes the cytoplasm also includes the nucleus, and other membrane-bound organelles.The cytoplasm is about 80% water and is usually colorless.

A vacuole is a membrane-bound organelle which is present in plant and fungal cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water containing inorganic and organic molecules including enzymes in solution, though in certain cases they may contain solids which have been engulfed. Vacuoles are formed by the fusion of multiple membrane vesicles and are effectively just larger forms of these. The organelle has no basic shape or size; its structure varies according to the requirements of the cell.

Paramecium is a genus of eukaryotic, unicellular ciliates, widespread in freshwater, brackish, and marine environments. Paramecia are often abundant in stagnant basins and ponds. Because some species are readily cultivated and easily induced to conjugate and divide, they have been widely used in classrooms and laboratories to study biological processes. Paramecium species are commonly studied as model organisms of the ciliate group and have been characterized as the "white rats" of the phylum Ciliophora.

Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.

Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer. Aquaporins have six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine–proline–alanine which form a barrel surrounding a central pore-like region that contains additional protein density. Because aquaporins are usually always open and are prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. Water can pass through the cell membrane through simple diffusion because it is a small molecule, and through osmosis, in cases where the concentration of water outside of the cell is greater than that of the inside. However, because water is a polar molecule this process of simple diffusion is relatively slow, and in tissues with high water permeability the majority of water passes through aquaporin.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion, active transport, osmosis, or reverse diffusion. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. Examples of channel/carrier proteins include the GLUT 1 uniporter, sodium channels, and potassium channels. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well.

In the kidney, the loop of Henle is the portion of a nephron that leads from the proximal convoluted tubule to the distal convoluted tubule. Named after its discoverer, the German anatomist Friedrich Gustav Jakob Henle, the loop of Henle's main function is to create a concentration gradient in the medulla of the kidney.

Cytolysis, or osmotic lysis, occurs when a cell bursts due to an osmotic imbalance that has caused excess water to diffuse into the cell. Water can enter the cell by diffusion through the cell membrane or through selective membrane channels called aquaporins, which greatly facilitate the flow of water. It occurs in a hypotonic environment, where water moves into the cell by osmosis and causes its volume to increase to the point where the volume exceeds the membrane's capacity and the cell bursts. The presence of a cell wall prevents the membrane from bursting, so cytolysis only occurs in animal and protozoa cells which do not have cell walls. The reverse process is plasmolysis.
An osmoreceptor is a sensory receptor primarily found in the hypothalamus of most homeothermic organisms that detects changes in osmotic pressure. Osmoreceptors can be found in several structures, including two of the circumventricular organs – the vascular organ of the lamina terminalis, and the subfornical organ. They contribute to osmoregulation, controlling fluid balance in the body. Osmoreceptors are also found in the kidneys where they also modulate osmolality.

An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts:

In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution.

Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution. The osmolarity of a solution is usually expressed as Osm/L, in the same way that the molarity of a solution is expressed as "M". Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of osmoles of solute particles per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration.
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.

Paramecium caudatum is a species of unicellular protist in the phylum Ciliophora. They can reach 0.33 mm in length and are covered with minute hair-like organelles called cilia. The cilia are used in locomotion and feeding. The species is very common, and widespread in marine, brackish and freshwater environments.

Osmosis is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential to a region of low water potential, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes to keep the body fluids from becoming too diluted or concentrated. Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis. The higher the osmotic pressure of a solution, the more water tends to move into it. Pressure must be exerted on the hypertonic side of a selectively permeable membrane to prevent diffusion of water by osmosis from the side containing pure water.

Amoeba is a genus of single-celled amoeboids in the family Amoebidae. The type species of the genus is Amoeba proteus, a common freshwater organism, widely studied in classrooms and laboratories.
Acidocalcisomes are rounded electron-dense acidic organelles, rich in calcium and polyphosphate and between 100 nm and 200 nm in diameter.
The rock dove, Columbia livia, has a number of special adaptations for regulating water uptake and loss.