
The cell cycle, or cell-division cycle, is the sequential series of events that take place in a cell that causes it to divide into two daughter cells. These events include the growth of the cell, duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.

Cytokinesis is the part of the cell division process and part of mitosis during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.

In cell biology, the cleavage furrow is the indentation of the cell's surface that begins the progression of cleavage, by which animal and some algal cells undergo cytokinesis, the final splitting of the membrane, in the process of cell division. The same proteins responsible for muscle contraction, actin and myosin, begin the process of forming the cleavage furrow, creating an actomyosin ring. Other cytoskeletal proteins and actin binding proteins are involved in the procedure.
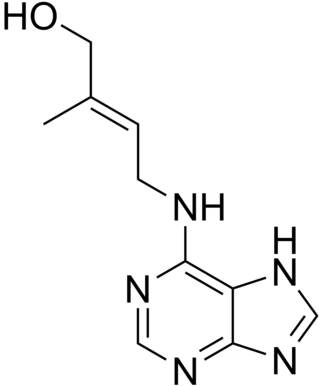
Cytokinins (CK) are a class of plant hormones that promote cell division, or cytokinesis, in plant roots and shoots. They are involved primarily in cell growth and differentiation, but also affect apical dominance, axillary bud growth, and leaf senescence.

Motor proteins are a class of molecular motors that can move along the cytoskeleton of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
The LIM kinases are a family of actin-binding kinases that phosphorylate members of the ADF/cofilin family of actin binding and filament severing proteins. The LIM kinase family is made up of two proteins: LIM kinase-1 (LIMK1) and LIM kinase-2 (LIMK2)

Aurora kinase B is a protein that functions in the attachment of the mitotic spindle to the centromere and in cytokinesis.

Rac GTPase-activating protein 1 is an enzyme that in humans is encoded by the RACGAP1 gene.
Kinesin-like protein KIF23 is a protein that in humans is encoded by the KIF23 gene.

Kinesin-like protein KIF3A is a protein that in humans is encoded by the KIF3A gene.

Citron Rho-interacting kinase is an enzyme that in humans is encoded by the CIT gene.

Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and cytokinesis. The ANLN gene in humans and the scraps gene in Drosophila encode Anillin. In 1989, anillin was first isolated in embryos of Drosophila melanogaster. It was identified as an F-actin binding protein. Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin antibody showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis. These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.

Protein Regulator of cytokinesis 1 (PRC1) is a protein that in humans is encoded by the PRC1 gene and is involved in cytokinesis.
Peptide signaling plays a significant role in various aspects of plant growth and development and specific receptors for various peptides have been identified as being membrane-localized receptor kinases, the largest family of receptor-like molecules in plants. Signaling peptides include members of the following protein families.
Kinesin family member 15 is a protein that in humans is encoded by the KIF15 gene.
PIN proteins are integral membrane proteins in plants that transport the anionic form of the hormone auxin across membranes. The discovery of the initial member of the PIN gene family, PIN1, occurred through the identification of the pin-formed1 (pin1) mutation in Arabidopsis thaliana. This mutation led to a stem that lacked almost all organs, including leaves and flowers.
Plant nucleus movement is the movement of the cell nucleus in plants by the cytoskeleton.
Lucas Andrew Staehelin was a retired Swiss-American cell biologist. He was professor emeritus at the University of Colorado Boulder.
Christoph Benning is a German–American plant biologist. He is an MSU Foundation Professor and University Distinguished Professor at Michigan State University. Benning's research into lipid metabolism in plants, algae and photosynthetic bacteria, led him to be named Editor-in-Chief of The Plant Journal in October 2008.














