Alkaloids are a broad class of naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.

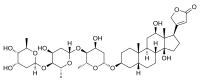
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses include treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants and milkweed plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment.

Digitalis is a genus of about 20 species of herbaceous perennial plants, shrubs, and biennials, commonly called foxgloves.

Thyme is a culinary herb consisting of the dried aerial parts of some members of the genus Thymus of flowering plants in the mint family Lamiaceae. Thymes are native to Eurasia and north Africa. Thymes have culinary, medicinal, and ornamental uses. The species most commonly cultivated and used for culinary purposes is Thymus vulgaris, native to Southeast Europe.
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists.

Secondary metabolites, also called specialised metabolites, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, archaea, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Phytochemicals are chemical compounds produced by plants, generally to help them resist fungi, bacteria and plant virus infections, and also consumption by insects and other animals. The name comes from Greek φυτόν (phyton) 'plant'. Some phytochemicals have been used as poisons and others as traditional medicine.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
Saponins are bitter-tasting, usually toxic plant-derived secondary metabolites. They are organic chemicals and have a foamy quality when agitated in water and a high molecular weight. They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but particularly in soapwort, a flowering plant, the soapbark tree, common corn-cockle, baby's breath and soybeans. They are used in soaps, medicines, fire extinguishers, dietary supplements, steroid synthesis, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Pharmacognosy is the study of crude drugs obtained from medicinal plants, animals, fungi, and other natural sources. The American Society of Pharmacognosy defines pharmacognosy as "the study of the physical, chemical, biochemical, and biological properties of drugs, drug substances, or potential drugs or drug substances of natural origin as well as the search for new drugs from natural sources".

Medicinal plants, also called medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. Plants synthesize hundreds of chemical compounds for various functions, including defense and protection against insects, fungi, diseases, against parasites and herbivorous mammals.
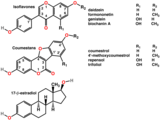
Isoflavones are substituted derivatives of isoflavone, a type of naturally occurring isoflavonoids, many of which act as phytoestrogens in mammals. Isoflavones occur in many plant species, but are especially high in soybeans.

Iridoids are a type of monoterpenoids in the general form of cyclopentanopyran, found in a wide variety of plants and some animals. They are biosynthetically derived from 8-oxogeranial. Iridoids are typically found in plants as glycosides, most often bound to glucose.
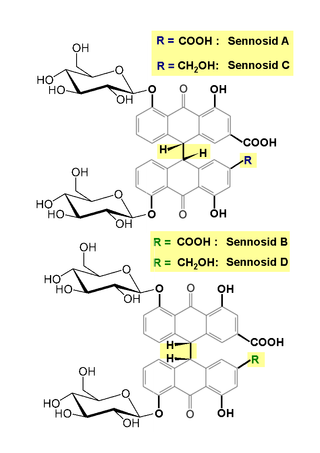
Senna glycoside, also known as sennoside or senna, is a medication used to treat constipation and empty the large intestine before surgery. The medication is taken by mouth or via the rectum. It typically begins working in around 30 minutes when given by rectum and within twelve hours when given by mouth. It is a weaker laxative than bisacodyl and castor oil.
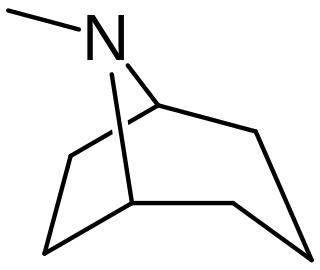
Tropane alkaloids are a class of bicyclic [3.2.1] alkaloids and secondary metabolites that contain a tropane ring in their chemical structure. Tropane alkaloids occur naturally in many members of the plant family Solanaceae. Certain tropane alkaloids such as cocaine and scopolamine are notorious for their psychoactive effects, related usage and cultural associations. Particular tropane alkaloids such as these have pharmacological properties and can act as anticholinergics or stimulants.

Secondary metabolism produces a large number of specialized compounds that do not aid in the growth and development of plants but are required for the plant to survive in its environment. Secondary metabolism is connected to primary metabolism by using building blocks and biosynthetic enzymes derived from primary metabolism. Primary metabolism governs all basic physiological processes that allow a plant to grow and set seeds, by translating the genetic code into proteins, carbohydrates, and amino acids. Specialized compounds from secondary metabolism are essential for communicating with other organisms in mutualistic or antagonistic interactions. They further assist in coping with abiotic stress such as increased UV-radiation. The broad functional spectrum of specialized metabolism is still not fully understood. In any case, a good balance between products of primary and secondary metabolism is best for a plant’s optimal growth and development as well as for its effective coping with often changing environmental conditions. Well known specialized compounds include alkaloids, polyphenols including flavonoids, and terpenoids. Humans use many of these compounds for culinary, medicinal and nutraceutical purposes.
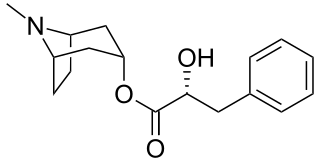
Littorine is a tropane alkaloid found in a variety of plants including Datura and Atropa belladonna. It is closely related in chemical structure to atropine, hyoscyamine, and scopolamine, which all share a common biosynthetic pathway.

Psychoactive plants are plants, or preparations thereof, that upon ingestion induce psychotropic effects. As stated in a reference work:
Psychoactive plants are plants that people ingest in the form of simple or complex preparations in order to affect the mind or alter the state of consciousness.

Cannabicitran (CBTC) is a phytocannabinoid first isolated in 1974 as a trace component of Cannabis sativa. Structurally related compounds can be found in some other plants. It is not psychoactive, but was found to reduce intraocular pressure in tests on rabbits, which may reflect agonist activity at the NAGly receptor that is known to be a target of many structurally related cannabinoids.