
Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
Anoikis is a form of programmed cell death that occurs in anchorage-dependent cells when they detach from the surrounding extracellular matrix (ECM). Usually cells stay close to the tissue to which they belong since the communication between proximal cells as well as between cells and ECM provide essential signals for growth or survival. When cells are detached from the ECM, there is a loss of normal cell–matrix interactions, and they may undergo anoikis. However, metastatic tumor cells may escape from anoikis and invade other organs.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.

The phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha, also called p110α protein, is a class I PI 3-kinase catalytic subunit. The human p110α protein is encoded by the PIK3CA gene.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit delta isoform also known as phosphoinositide 3-kinase (PI3K) delta isoform or p110δ is an enzyme that in humans is encoded by the PIK3CD gene.

Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing alpha polypeptide is an enzyme that in humans is encoded by the PIK3C2A gene.

Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing beta polypeptide is an enzyme that in humans is encoded by the PIK3C2B gene.

LY294002 is a morpholine-containing chemical compound that is a potent inhibitor of numerous proteins, and a strong inhibitor of phosphoinositide 3-kinases (PI3Ks). It is generally considered a non-selective research tool, and should not be used for experiments aiming to target PI3K uniquely.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

Phosphoinositide 3-kinase inhibitors are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy. While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable.

The PI3K/AKT/mTOR pathway is an intracellular signaling pathway important in regulating the cell cycle. Therefore, it is directly related to cellular quiescence, proliferation, cancer, and longevity. PI3K activation phosphorylates and activates AKT, localizing it in the plasma membrane. AKT can have a number of downstream effects such as activating CREB, inhibiting p27, localizing FOXO in the cytoplasm, activating PtdIns-3ps, and activating mTOR which can affect transcription of p70 or 4EBP1. There are many known factors that enhance the PI3K/AKT pathway including EGF, shh, IGF-1, insulin, and CaM. Both leptin and insulin recruit PI3K signalling for metabolic regulation. The pathway is antagonized by various factors including PTEN, GSK3B, and HB9.
Lewis C. Cantley is an American cell biologist and biochemist who has made significant advances to the understanding of cancer metabolism. Among his most notable contributions are the discovery and study of the enzyme PI-3-kinase, now known to be important to understanding cancer and diabetes mellitus. He is currently Meyer Director and Professor of Cancer Biology at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine in New York City. He was formerly a professor in the Departments of Systems Biology and Medicine at Harvard Medical School, and the Director of Cancer Research at the Beth Israel Deaconess Medical Center, in Boston, Massachusetts. In 2016, he was elected Chairman of the Board for the Hope Funds for Cancer Research.
Edelfosine is a synthetic alkyl-lysophospholipid (ALP). It has antineoplastic (anti-cancer) effects.
Dalotuzumab is an anti-IGF1 receptor (IGF1R) humanized monoclonal antibody designed for the potential treatment of various cancers. Common adverse effects include hyperglycemia, nausea, vomiting, and fatigue. Dalotuzumab was developed by Merck and Co., Inc.

mTOR inhibitors are a class of drugs that inhibit the mammalian target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.
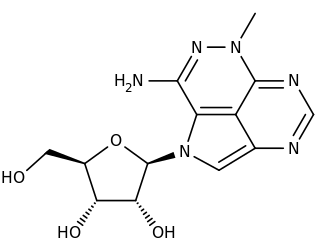
Triciribine is a cancer drug which was first synthesized in the 1970s and studied clinically in the 1980s and 1990s without success. Following the discovery in the early 2000s that the drug would be effective against tumours with hyperactivated Akt, it is now again under consideration in a variety of cancers. As PTX-200, the drug is currently in two early stage clinical trials in breast cancer and ovarian cancer being conducted by the small molecule drug development company Prescient Therapeutics.

Buparlisib is an experimental anti-cancer medication. It is a small molecule orally-available pan-class I phosphoinositide 3-kinase (PI3K) inhibitor. Buparlisib was under investigation as a treatment for advanced breast cancer but was abandoned due to negative results. It is still under investigation as a potential treatment for head and neck squamous cell carcinoma (HNSCC).

Gedatolisib (PF-05212384) is an experimental drug for treatment of cancer in development by Celcuity, Inc. The mechanism of action is accomplished by binding the different p110 catalytic subunit isoforms of PI3K and the kinase site of mTOR.














