Related Research Articles
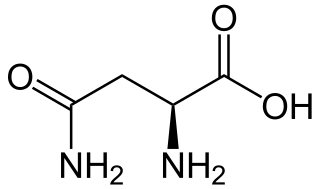
Asparagine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain carboxamide, classifying it as a polar, aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
The year 1890 in science and technology involved some significant events, listed below.

Ferdinand Frédéric Henri Moissan was a French chemist and pharmacist who won the 1906 Nobel Prize in Chemistry for his work in isolating fluorine from its compounds. Moissan was one of the original members of the International Atomic Weights Committee.
In organic chemistry, the Knoevenagel condensation reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation.
The Bischler–Möhlau indole synthesis, also often referred to as the Bischler indole synthesis, is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline; it is named after August Bischler and Richard Möhlau .

Hippuric acid is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenolic compounds. The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
The Pechmann condensation is a synthesis of coumarins, starting from a phenol and a carboxylic acid or ester containing a β-carbonyl group. The condensation is performed under acidic conditions. The mechanism involves an esterification/transesterification followed by attack of the activated carbonyl ortho to the oxygen to generate the new ring. The final step is a dehydration, as seen following an aldol condensation. It was discovered by the German chemist Hans von Pechmann .

The Schiff test is an early organic chemistry named reaction developed by Hugo Schiff, and is a relatively general chemical test for detection of many organic aldehydes that has also found use in the staining of biological tissues. The Schiff reagent is the reaction product of a dye formulation such as fuchsin and sodium bisulfite; pararosaniline and new fuchsin are not dye alternatives with comparable detection chemistry.

The iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. The iodine clock reaction exists in several variations, which each involve iodine species and redox reagents in the presence of starch. Two colourless solutions are mixed and at first there is no visible reaction. After a short time delay, the liquid suddenly turns to a shade of dark blue due to the formation of a triiodide–starch complex. In some variations, the solution will repeatedly cycle from colorless to blue and back to colorless, until the reagents are depleted.
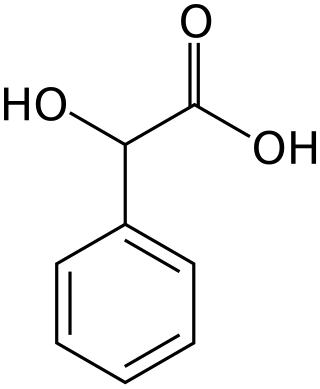
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.
The Leuckart reaction is the chemical reaction that converts aldehydes or ketones to amines. The reaction is an example of reductive amination. The reaction, named after Rudolf Leuckart, uses either ammonium formate or formamide as the nitrogen donor and reducing agent. It requires high temperatures, usually between 120 and 130 °C; for the formamide variant, the temperature can be greater than 165 °C.

Phenyl salicylate, or salol, is the organic compound with the formula C6H5O2C6H4OH. It is a white solid. It is occasionally used in sunscreens and as an antiseptic.
The Schotten–Baumann reaction is a method to synthesize amides from amines and acid chlorides:

Hans Heinrich Landolt was a Swiss chemist who discovered iodine clock reaction. He is also one of the founders of Landolt–Börnstein database. He tested law of mass conservation which was given by Lavoisier.

Nikolai Aleksandrovich Menshutkin was a Russian chemist who discovered the process of converting a tertiary amine to a quaternary ammonium salt via the reaction with an alkyl halide, now known as the Menshutkin reaction.
Trisilane is the silane with the formula H2Si(SiH3)2. A liquid at standard temperature and pressure, it is a silicon analogue of propane. In contrast with propane, however, trisilane ignites spontaneously in air.

Carl Wilhelm Bernhard Scheibler was a German chemist. Scheibler's research focused on sugar, including the technical chemistry of sugar production and the composition of molasses.

Fluorine is a relatively new element in human applications. In ancient times, only minor uses of fluorine-containing minerals existed. The industrial use of fluorite, fluorine's source mineral, was first described by early scientist Georgius Agricola in the 16th century, in the context of smelting. The name "fluorite" derives from Agricola's invented Latin terminology. In the late 18th century, hydrofluoric acid was discovered. By the early 19th century, it was recognized that fluorine was a bound element within compounds, similar to chlorine. Fluorite was determined to be calcium fluoride.
References
- ↑ Moissan, H. (1886). "Action d'un courant électrique sur l'acide fluorhydrique anhydre". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 102: 1543–1544.
- ↑ Moissan, H. (1886). "Sur la décomposition de l'acide fluorhydrique par un courant électrique". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 103: 202.
- ↑ Landolt, H. (1886). "Ueber die Zeitdauer der Reaction zwischen Jodsäure und schwefliger Säure" [On the duration of the reaction between iodic acid and sulfurous acid]. Berichte der Deutschen Chemischen Gesellschaft (in German). 19 (1): 1317–1365. doi:10.1002/cber.188601901293.
- ↑ Landolt, H. (1887). "Ueber die Zeitdauer der Reaction zwischen Jodsäure und schwefliger Säure [Part 2]" [On the duration of the reaction between iodic acid and sulfurous acid]. Berichte der Deutschen Chemischen Gesellschaft (in German). 20 (1): 745–760. doi:10.1002/cber.188702001173.
- ↑ Choice and Chance: An Elementary Treatise on Permutations, Combinations, and Probability. 1886 edn.
- ↑ "Sur une forme particulière d'atrophie musculaire progressive, souvent familiale débutant par les pieds et les jambes et atteignant plus tard les mains". Revue médicale. 6: 97–138. 1886.
- ↑ The Peroneal Type of Progressive Muscular Atrophy. Lewis. 1886.
- ↑ Enersen, Ole Daniel. "Charcot-Marie-Tooth disease". Whonamedit? . Archived from the original on 14 May 2011. Retrieved 2011-04-12.
- ↑ Hunt, T. J.; Thienhaus, O.; Ellwood, A. (July 2008). "The mirror lies: Body dysmorphic disorder". American Family Physician. 78 (2): 217–22. PMID 18697504.
- ↑ Carroll, Deirdre H.; Scahill, Larry; Phillips, Katharine A. (April 2002). "Current concepts in body dysmorphic disorder". Archives of Psychiatric Nursing. 16 (2): 72–79. doi:10.1053/apnu.2002.32109. PMID 11925574.
- ↑ Pepper, Sarah (October 1992). "Allinson's Staff of Life – Health Without Medicine in the 1890s". History Today . 42 (10): 30–35. Retrieved 2012-01-26.
- ↑ Patent DRP 39-367.
- ↑ "The Akroyd Oil Engine". Ray Hooley's – Ruston-Hornsby – Engine Pages. Archived from the original on 2011-05-24. Retrieved 2011-05-11.
- ↑ "Schuyler Wheeler". IEEE Global History Network. IEEE . Retrieved 2012-01-25.
- ↑ "Copley Medal | British scientific award". Encyclopedia Britannica. Retrieved 23 July 2020.