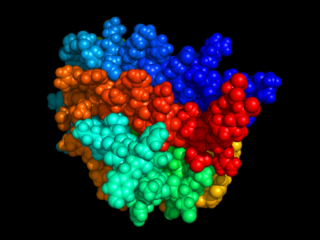
Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease and mouth disease.

Amgen Inc. is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, As of 2022, Amgen has approximately 24,000 staff in total.
Takeda Oncology is a biopharmaceutical company based in Cambridge, Massachusetts. It is a fully owned subsidiary of Takeda Pharmaceutical.

Ortho-McNeil Pharmaceutical was a pharmaceutical company based in Raritan, New Jersey, that was formed from the merger of Ortho Pharmaceutical and McNeil Pharmaceutical in 1993. Both of these pharmaceutical companies were pioneers and leaders in areas such as pain management, acid reflux disease, and infectious diseases.

Desvenlafaxine, sold under the brand name Pristiq among others, is a medication used to treat depression. It is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class and is taken by mouth. It is recommended that the need for further treatment be occasionally reassessed. It may be less effective than its parent compound venlafaxine, although some studies have found comparable efficacy.

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its name, the company branched out into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap.

Erythropoiesis-stimulating agents (ESA) are medications which stimulate the bone marrow to make red blood cells. They are used to treat anemia due to end stage kidney disease, chemotherapy, major surgery, or certain treatments in HIV/AIDS. In these situations they decrease the need for blood transfusions. The different agents are more or less equivalent. They are given by injection.

Glenmark Pharmaceuticals Limited is an Indian multinational pharmaceutical company headquartered in Mumbai, India.
Peginesatide, developed by Affymax and Takeda, is an erythropoietic agent, a functional analog of erythropoietin.
Evenamide is a selective voltage-gated sodium channel blocker, including subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia. The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression. It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.

Centanafadine (INN) (former developmental code name EB-1020) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) that began its development with Euthymics Bioscience after they acquired DOV Pharmaceutical. It was developed as a treatment for attention-deficit hyperactivity disorder (ADHD) and inhibits the reuptake of norepinephrine, dopamine, and serotonin with an IC50 ratio of 1:6:14, respectively. In 2011, Euthymics Bioscience spun off its development of centanafadine to a new company called Neurovance. In March 2017, Otsuka Pharmaceutical acquired Neurovance and the rights to centanafadine. As of January 2018, Otsuka's pipeline indicates it is in Phase II and III clinical trials for a number of different applications to medical conditions.
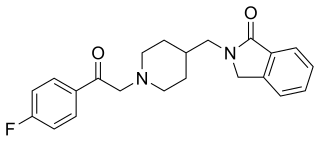
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist under development by Minerva Neurosciences for the treatment of schizophrenia. One of its metabolites also has some affinity for the H1 receptor. Pre-clinical findings provide evidence of the effect of roluperidone on brain-derived neurotrophic factor ("BDNF"), which has been associated with neurogenesis, neuroplasticity, neuroprotection, synapse regulation, learning and memory.
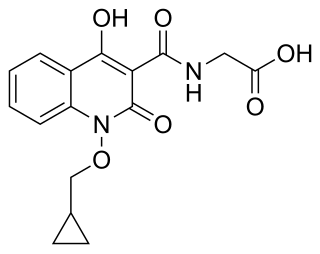
Desidustat is a drug for the treatment of anemia of chronic kidney disease. This drug with the brand name Oxemia is discovered and developed by Zydus Life Sciences. Desidustat reduces the requirement of recombinant erythropoietin (EPO) in anemia, and decreases EPO-resistance, by reducing IL-6, IL-1β, and anti-EPO antibodies. The subject expert committee of CDSCO has recommended the grant of permission for manufacturing and marketing of Desidustat 25 mg and 50 mg tablets in India, based on some conditions related to package insert, phase 4 protocols, prescription details, and GCP. Clinical trials on desidustat have been done in India and Australia. In a Phase 2, randomized, double-blind, 6-week, placebo-controlled, dose-ranging, safety and efficacy study, a mean hemoglobin increase of 1.57, 2.22, and 2.92 g/dL in desidustat 100, 150, and 200 mg arms, respectively, was observed. The Phase 3 clinical trials were conducted in chronic kidney disease patients which were not on dialysis as well as on dialysis. Desidustat is developed for the treatment of anemia as an oral tablet, where currently injections of erythropoietin and its analogues are drugs of choice. Desidustat is a HIF prolyl-hydroxylase inhibitor. In preclinical studies, effects of desidustat was assessed in normal and nephrectomized rats, and in chemotherapy-induced anemia. Desidustat demonstrated hematinic potential by combined effects on endogenous erythropoietin release and efficient iron utilization. Desidustat can also be useful in treatment of anemia of inflammation since it causes efficient erythropoiesis and hepcidin downregulation. Desidustat has been shown to have significant effect in the treatment of complement-mediated diseases. Complement activation-induced membrane attack complex (MAC) formation and Factor B activity were also reduced by desidustat treatment. Owing to this mechanism, desidustat can be an effective therapy against membranous nephropathy and retinal degeneration, since it specifically inhibited alternative complement system, without affecting the lectin-, or classical complement pathway. In January 2020, Zydus entered into licensing agreement with China Medical System (CMS) Holdings for development and commercialization of desidustat in Greater China. Under the license agreement, CMS will pay Zydus an initial upfront payment, regulatory milestones, sales milestones and royalties on net sales of the product. CMS will be responsible for development, registration and commercialization of desidustat in Greater China. National Medical Products Administration of China (NMPA) accepted the new drug application for desidustat on 23 April 2024. It has been observed that desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines like IL-6 and oxidative stress. A clinical trial to evaluate the efficacy and safety of desidustat tablet for the management of COVID-19 patients is ongoing in Mexico, wherein desidustat has shown to prevent acute respiratory distress syndrome (ARDS) by inhibiting IL-6. Zydus has also received approval from the US FDA to initiate clinical trials of desidustat in chemotherapy Induced anemia (CIA). Desidustat was successfully introduced as an alternative treatment in patient of endogenous erythropoietin (EPO)-induced pure red cell aplasia (PRCA) due to anti-EPO antibodies. This led to a substantial and sustained improvement in hemoglobin levels, emphasizing the crucial role of desidustat intervention in EPO-induced PRCA cases. Zydus Lifesciences and Sun Pharmaceuticals have entered an agreement in October 2023 to co-market Desidustat. Sun Pharma will sell the drug as Rytstat, while Zydus will continue to sell it as Oxemia.
XP-21279 is a sustained-release levodopa (L-DOPA) prodrug and hence a dopamine precursor and non-selective dopamine receptor agonist which was under development for the treatment of Parkinson's disease. It is taken by mouth.
Arcarine (ORM-11984) is a selective androgen receptor modulator (SARM) developed by Orion Corporation, a Finnish pharmaceutical company. It belongs to a class of drugs designed to have tissue-selective androgenic effects, potentially offering the benefits of androgens while minimizing unwanted side effects. Arcarine was investigated for the treatment of various conditions, including benign prostatic hyperplasia, hypogonadism, and osteoporosis. The compound reached Phase I clinical trials before development was discontinued. Like other SARMs, Arcarine was developed to potentially provide anabolic effects in muscle and bone tissue while having reduced androgenic effects in other tissues, such as the prostate.
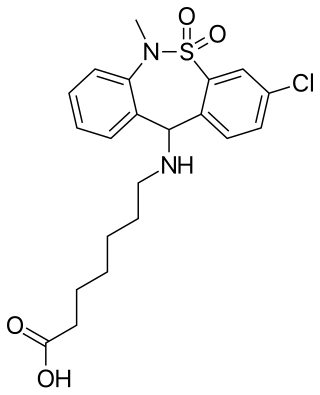
Tianeptine/naloxone, or naloxone/tianeptine, is an extended-release combination of tianeptine, an atypical μ-opioid receptor agonist, and naloxone, an orally inactive μ-opioid receptor antagonist, which was under development for the treatment of major depressive disorder, post-traumatic stress disorder (PTSD), and neurocognitive dysfunction associated with corticosteroid use but was never marketed.
OPC-64005 is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI), or "triple reuptake inhibitor" (TRI), which is under development for the treatment of major depressive disorder. It was also under development for the treatment of attention deficit hyperactivity disorder (ADHD), but development for this indication was discontinued. It is taken by mouth.

ENX-104, also known as deuterated nemonapride enantiomer, is a selective dopamine D2 and D3 receptor antagonist which is under development for the treatment of major depressive disorder. It is specifically under development for the treatment of major depressive disorder characterized by anhedonia. The drug is being developed for use at low doses to preferentially block presynaptic dopamine D2 and D3 autoreceptors and hence to enhance rather than inhibit dopaminergic neurotransmission. It is taken by mouth.
ACD856, or ACD-856, is a tropomyosin receptor kinase TrkA, TrkB, and TrkC positive allosteric modulator which is under development for the treatment of Alzheimer's disease, depressive disorders, sleep disorders, and traumatic brain injuries. It is taken by mouth.
SP-624 is a sirtuin-6 (SIRT6) activator which is under development for the treatment of major depressive disorder and schizophrenia. It is taken by mouth. As of August 2024, SP-624 is in phase 2 clinical trials for major depressive disorder and is in phase 1/2 clinical trials for schizophrenia. It is under development by Sirtsei Pharmaceuticals and Arrivo BioVentures. The drug is said to be a small molecule, but its chemical structure does not yet seem to have been disclosed.











