
The Orbiliaceae are a family of saprobic sac fungi. It is the only family in the monotypic class Orbiliomycetes and the monotypic order Orbiliales. The family was first described by John Axel Nannfeldt in 1932 and now contains 288 species in 12 genera. Members of this family have a widespread distribution, but are more prevalent in temperate regions. Some species in the Orbiliaceae are carnivorous fungi, and have evolved a number of specialized mechanisms to trap nematodes.

Cookeina is a genus of cup fungi in the family Sarcoscyphaceae, members of which may be found in tropical and subtropical regions of the world. Species may be found on fallen branches of angiosperms, trunks, and sometimes on fruits. The Temuans of Peninsular Malaysia are reported to use certain species from this genus as food, and also as a bait for fishing, where it is rubbed against the hook.

Chorioactis is a genus of fungi that contains the single species Chorioactis geaster. The mushroom is commonly known as the devil's cigar or the Texas star in the United States, while in Japan it is called kirinomitake (キリノミタケ). This extremely rare mushroom is notable for its unusual appearance and disjunct distribution; it is found only in select locales in Texas and Japan. The fruit body, which grows on the stumps or dead roots of cedar elms or dead oaks, somewhat resembles a dark brown or black cigar before it splits open radially into a starlike arrangement of four to seven leathery rays. The interior surface of the fruit body bears the spore-bearing tissue known as the hymenium, and is colored white to brown, depending on its age. The fruit body opening can be accompanied by a distinct hissing sound and the release of a smoky cloud of spores.
Lithoglypha is a fungal genus in the family Acarosporaceae. It is monotypic, containing the single species Lithoglypha aggregata, a saxicolous (rock-dwelling), crustose lichen found in South Africa.
Aquamarina is a fungal genus in the class Dothideomycetes. It is a monotypic genus, containing the single marine species Aquamarina speciosa, originally found in North Carolina, and distributed in the Atlantic Coast of the United States. The bluish-green species fruits exclusively in the lower parts of dying culms of the saltmarsh plant Juncus roemerianus.

Zeus is a fungal genus within the family Rhytismataceae. It is a monotypic genus, containing the single species Zeus olympius, originally discovered in 1987 on Mount Olympus in Greece. Fruit bodies are yellow discs that grow in the decaying wood of Bosnian pine trees.

Sarcoscypha occidentalis, commonly known as the stalked scarlet cup or the western scarlet cup, is a species of fungus in the family Sarcoscyphaceae of the Pezizales order. Phylogenetic analysis has shown that it is most closely related to other Sarcoscypha species that contain large oil droplets in their spores. S. occidentalis has an imperfect form, classified as Molliardiomyces occidentalis.

Galiella rufa, commonly known as the rubber cup, the rufous rubber cup, or the hairy rubber cup, is a species of fungus in the family Sarcosomataceae. It produces cup-shaped fruit bodies with the texture of tough, gelatinous rubber, with a rough, blackish-brown, felt-like outer surface and a smooth reddish-brown inner surface.
Massarina carolinensis is a species of fungus in the Lophiostomataceae family. The species is found exclusively on the lower parts of the culms of the saltmarsh Juncus roemerianus on the Atlantic Coast of North Carolina.
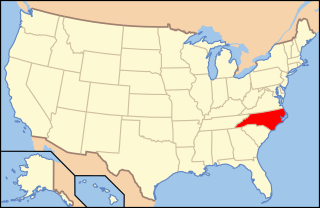
Paraphaeosphaeria pilleata is a species of fungus in the Lophiostomataceae family. The species fruits exclusively in the lower parts of the culms of the black needlerush. It is found on the Atlantic Coast of North Carolina.

Glutinoglossum glutinosum, commonly known as the viscid black earth tongue or the glutinous earthtongue, is a species of fungus in the family Geoglossaceae. Widely distributed in the Northern Hemisphere, it has been found in northern Africa, Asia, Europe, and North America. Although previously thought to exist in Australasia, collections made from these locations have since been referred to new species. G. glutinosum is a saprophytic species that grows on soil in moss or in grassy areas. The smooth, nearly black, club-shaped fruitbodies grow to heights ranging from 1.5 to 5 cm. The head is up to 0.7 cm (0.3 in) long, and the stipes are sticky. Several other black earth tongue species are quite similar in external appearance, and many can be reliably distinguished only by examining differences in microscopic characteristics, such as spores, asci, and paraphyses. First described in 1796 as a species of Geoglossum, the fungus has gone through several changes of genera in its taxonomic history. It was placed in its current genus, Glutinoglossum, in 2013.
Nanostictis caucasica is a species of lichenicolous (lichen-eating) fungus in the family Stictidaceae. It is known to occur only in a single locality in the North Caucasus region of Southern Russia, where it grows parasitically on the foliose lichen Parmelia sulcata.
Asterothyrium vezdae is a species of foliicolous (leaf-dwelling) lichen in the family Gomphillaceae. It is found in Bolivia, where it grows on the leaves of vascular plants in the Amazon rainforest. The lichen is distinguished from its closest relative, Asterothyrium octomerum, by the larger number of septa in its ascospores, and its and black apothecia.
Candelariella lichenicola is a rare species of lichenicolous (lichen-dwelling) fungus in the family Candelariaceae. This species was first found in Sonora, Mexico, and is characterised by its distinct spore shape and chemical composition. It is typically found growing on the lichen species Candelina submexicana, and while not widespread, it contributes to the ecological diversity of the regions it inhabits.
Podotara is a fungal genus in the family Pilocarpaceae. It is a monotypic genus, containing the single species Podotara pilophoriformis, an uncommon foliicolous (leaf-dwelling), crustose lichen that grows on Podocarpus totara, a species of podocarp tree endemic to New Zealand. Both the genus and the species were proposed in 1996.
Caloplaca nothocitrina is a species of saxicolous (rock-dwelling), crustose lichen in the family Teloschistaceae. Its thallus is up to 8 mm in diameter and deep yellow in colour. It comprises small dispersed areoles, occasional concave soralia, and circular apothecia with a bright yellow margin and a dull dark yellowish or brownish disc.

Flavoplaca oasis is a species of saxicolous (rock-dwelling), crustose lichen in the family Teloschistaceae. It is widely distributed across Europe, and has been reported in Western Asia, China, and North Africa.
Elixjohnia jackelixii is a species of saxicolous (rock-dwelling), crustose lichen in the family Teloschistaceae. It is found in Australia and New Zealand. The lichen is characterised by its unique multilayered appearance with outer sterile rings that are brownish or greenish-yellow and inner areoles that are whitish, yellowish, or greyish, often cracked to reveal the medulla underneath. Its fruiting bodies, or apothecia, are typically attached directly to the thallus and vary in colour and shape.
Nitidochapsa is a genus of lichen-forming fungi in the family Graphidaceae. It has five species of corticolous (bark-dwelling), crustose lichens.
Tricharia nigriuncinata is a species of foliicolous (leaf-dwelling) lichen in the family Gomphillaceae. It is found in East Africa.











