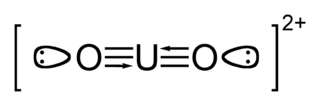A coordination complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes. A coordination complex whose centre is a metal atom is called a metal complex of d block element.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad of organic compounds, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
Compounds of carbon are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−
4.Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used. The prefix of the word 'amphoteric' is derived from a Greek prefix amphi-, which means both.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.

Zinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms spectacular opaque clouds of titanium dioxide (TiO2) and hydrated hydrogen chloride. It is sometimes referred to as "tickle" or "tickle 4" due to the phonetic resemblance of its molecular formula (TiCl4) to the word.
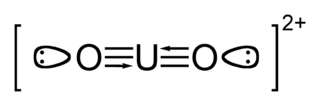
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Zinc cyanide is the inorganic compound with the formula Zn(CN)2. It is a white solid that is used mainly for electroplating zinc but also has more specialized applications for the synthesis of organic compounds.

Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2. This white, crystalline solid is highly deliquescent and is typically encountered as a hexahydrate Zn(NO3)2•6H2O. It is soluble in both water and alcohol.
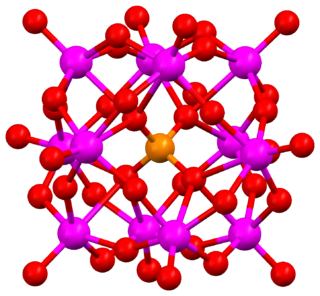
Keggin structure is the best known structural form for heteropoly acids. It is the structural form of α-Keggin anions, which have a general formula of [XM12O40]n−, where X is the heteroatom (most commonly are P5+, Si4+, or B3+), M is the addenda atom (most common are molybdenum and tungsten), and O represents oxygen. The structure self-assembles in acidic aqueous solution and is the most stable structure of polyoxometalate catalysts.
Thiophosphates are chemical compounds and anions with the general chemical formula PS
4−xO3−
x and related derivatives where organic groups are attached to one or more O or S. Thiophosphates feature tetrahedral phosphorus(V) centers.
Metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorates. They have the general stoichiometry [M(H2O)n]z+. Their behavior underpins many aspects of environmental, biological, and industrial chemistry. This article focuses on complexes where water is the only ligand ("homoleptic aquo complexes"), but of course many complexes are known to consist of a mix of aquo and other ligands.
A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [M(H2O)n]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in periods 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have a solvation number of 8 or 9. The strength of the bonds between the metal ion and water molecules in the primary solvation shell increases with the electrical charge, z, on the metal ion and decreases as its ionic radius, r, increases. Aqua ions are subject to hydrolysis. The logarithm of the first hydrolysis constant is proportional to z2/r for most aqua ions.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.
Compounds of nickel are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Metal peroxides are metal-containing compounds with ionically- or covalently-bonded peroxide (O2−
2) groups. This large family of compounds can be divided into ionic and covalent peroxide. The first class mostly contains the peroxides of the alkali and alkaline earth metals whereas the covalent peroxides are represented by such compounds as hydrogen peroxide and peroxymonosulfuric acid (H2SO5). In contrast to the purely ionic character of alkali metal peroxides, peroxides of transition metals have a more covalent character.



![Structure of solid basic zinc acetate, [Zn
4(m -O)(e -O
2CCH
3)
6] BasicZnAcetate.png](http://upload.wikimedia.org/wikipedia/commons/thumb/9/9c/BasicZnAcetate.png/330px-BasicZnAcetate.png)