 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
PubChem CID | |
| |
| |
| Properties | |
| C9H6Fe2O6S2 | |
| Molar mass | 385.83 |
| Appearance | red solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
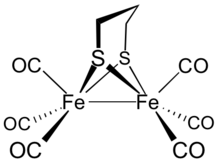
Diiron propanedithiolate hexacarbonyl is the organoiron complex with the formula Fe2(S2C3H6)(CO)6. It is a red diamagnetic solid. [1] It adopts a symmetrical structure with six terminal CO ligands. [2] The complex is a precursor to hydrogenase mimics. [3]
It is prepared by the reaction of 1,3-propanedithiol with triiron dodecacarbonyl:
- 2 Fe3(CO)12 + 3 C3H6(SH)2 → 3 Fe2(S2C3H6)(CO)6 + 3 H2 + 6 CO
In general, the CO ligands can be substituted by cyanide, phosphines, isocyanides, N-heterocyclic carbenes, and other donor ligands. Monosubstitution can be achieved through an in situ generation of the acetonitrile complex. [4] [5]
Upon irradiation of Fe2(S2C3H6)(CO)6 with ultraviolet (UV) light, CO-photolysis occurs with the transient formation of the unsaturated species followed by the formation of the solvent adduct. [6]