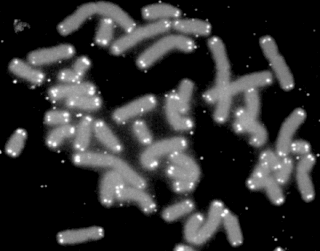
A telomere is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Telomeres are a widespread genetic feature most commonly found in eukaryotes. In most, if not all species possessing them, they protect the terminal regions of chromosomal DNA from progressive degradation and ensure the integrity of linear chromosomes by preventing DNA repair systems from mistaking the very ends of the DNA strand for a double-strand break.
Life extension is the concept of extending the human lifespan, either modestly through improvements in medicine or dramatically by increasing the maximum lifespan beyond its generally-settled biological limit of around 125 years. Several researchers in the area, along with "life extensionists", "immortalists", or "longevists", postulate that future breakthroughs in tissue rejuvenation, stem cells, regenerative medicine, molecular repair, gene therapy, pharmaceuticals, and organ replacement will eventually enable humans to have indefinite lifespans through complete rejuvenation to a healthy youthful condition (agerasia). The ethical ramifications, if life extension becomes a possibility, are debated by bioethicists.

Telomerase, also called terminal transferase, is a ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3' end of telomeres. A telomere is a region of repetitive sequences at each end of the chromosomes of most eukaryotes. Telomeres protect the end of the chromosome from DNA damage or from fusion with neighbouring chromosomes. The fruit fly Drosophila melanogaster lacks telomerase, but instead uses retrotransposons to maintain telomeres.

Elizabeth Helen Blackburn, is an Australian-American Nobel laureate who is the former president of the Salk Institute for Biological Studies. In 1984, Blackburn co-discovered telomerase, the enzyme that replenishes the telomere, with Carol W. Greider. For this work, she was awarded the 2009 Nobel Prize in Physiology or Medicine, sharing it with Carol W. Greider and Jack W. Szostak, becoming the first Australian woman Nobel laureate.
Biological immortality is a state in which the rate of mortality from senescence is stable or decreasing, thus decoupling it from chronological age. Various unicellular and multicellular species, including some vertebrates, achieve this state either throughout their existence or after living long enough. A biologically immortal living being can still die from means other than senescence, such as through injury, poison, disease, predation, lack of available resources, or changes to environment.

The Hayflick limit, or Hayflick phenomenon, is the number of times a normal somatic, differentiated human cell population will divide before cell division stops. However, this limit does not apply to stem cells.
Rejuvenation is a medical discipline focused on the practical reversal of the aging process.

Dyskeratosis congenita (DKC), also known as Zinsser-Engman-Cole syndrome, is a rare progressive congenital disorder with a highly variable phenotype. The entity was classically defined by the triad of abnormal skin pigmentation, nail dystrophy, and leukoplakia of the oral mucosa, and MDS/AML, but these components do not always occur. DKC is characterized by short telomeres. Some of the manifestations resemble premature ageing and cognitive impairment can be a feature. The disease initially mainly affects the skin, but a major consequence is progressive bone marrow failure which occurs in over 80%, causing early mortality.
Enquiry into the evolution of ageing, or aging, aims to explain why a detrimental process such as ageing would evolve, and why there is so much variability in the lifespans of organisms. The classical theories of evolution suggest that environmental factors, such as predation, accidents, disease, and/or starvation, ensure that most organisms living in natural settings will not live until old age, and so there will be very little pressure to conserve genetic changes that increase longevity. Natural selection will instead strongly favor genes which ensure early maturation and rapid reproduction, and the selection for genetic traits which promote molecular and cellular self-maintenance will decline with age for most organisms.

Carolyn Widney Greider is an American molecular biologist and Nobel laureate. She joined the University of California, Santa Cruz as a Distinguished Professor in the department of molecular, cell, and developmental biology in October 2020.
The following outline is provided as an overview of and topical guide to life extension:

Telomerase reverse transcriptase is a catalytic subunit of the enzyme telomerase, which, together with the telomerase RNA component (TERC), comprises the most important unit of the telomerase complex.
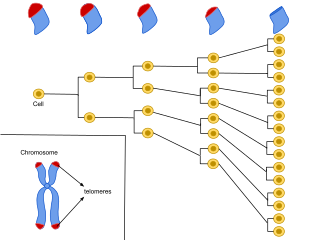
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.
The anti-aging movement is a social movement devoted to eliminating or reversing aging, or reducing the effects of it. A substantial portion of the attention of the movement is on the possibilities for life extension, but there is also interest in techniques such as cosmetic surgery which ameliorate the effects of aging rather than delay or defeat it.
BioViva is an American biotechnology gene therapy company, based in Bainbridge Island, Washington, researching treatments to interfere in the aging process in humans.

Life Length is a biotechnology company. Located in Madrid, it provides telomere diagnostics as well as telomerase measurement.
The disposable soma theory of aging states that organisms age due to an evolutionary trade-off between growth, reproduction, and DNA repair maintenance. Formulated by Thomas Kirkwood, the disposable soma theory explains that an organism only has a limited amount of resources that it can allocate to its various cellular processes. Therefore, a greater investment in growth and reproduction would result in reduced investment in DNA repair maintenance, leading to increased cellular damage, shortened telomeres, accumulation of mutations, compromised stem cells, and ultimately, senescence. Although many models, both animal and human, have appeared to support this theory, parts of it are still controversial. Specifically, while the evolutionary trade-off between growth and aging has been well established, the relationship between reproduction and aging is still without scientific consensus, and the cellular mechanisms largely undiscovered.
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. The hallmarks of aging are the types of biochemical changes that occur in all organisms that experience biological aging and lead to a progressive loss of physiological integrity, impaired function and, eventually, death. They were first listed in a landmark paper in 2013 to conceptualize the essence of biological aging and its underlying mechanisms.
This timeline lists notable events in the history of research into senescence or biological aging, including the research and development of life extension methods, brain aging delay methods and rejuvenation.
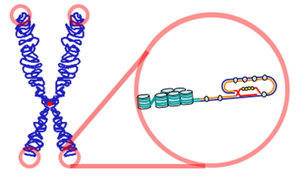
The relationship between telomeres and longevity and changing the length of telomeres is one of the new fields of research on increasing human lifespan and even human immortality. Telomeres are sequences at the ends of chromosomes that shorten with each cell division and determine the lifespan of cells. The telomere was first discovered by biologist Hermann Joseph Muller in the early 20th century. However, experiments by Elizabeth Blackburn, Carol Greider, and Jack Szostak in the 1980s led to the successful discovery of telomerase and a better understanding of telomeres.