
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth. Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development.
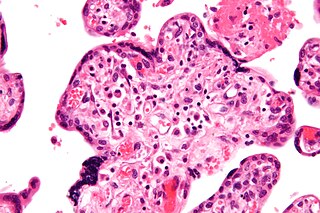
Intrauterine growth restriction (IUGR), or fetal growth restriction, is the poor growth of a fetus while in the womb during pregnancy. IUGR is defined by clinical features of malnutrition and evidence of reduced growth regardless of an infant's birth weight percentile. The causes of IUGR are broad and may involve maternal, fetal, or placental complications.

Placenta praevia is when the placenta attaches inside the uterus but in a position near or over the cervical opening. Symptoms include vaginal bleeding in the second half of pregnancy. The bleeding is bright red and tends not to be associated with pain. Complications may include placenta accreta, dangerously low blood pressure, or bleeding after delivery. Complications for the baby may include fetal growth restriction.
Oligohydramnios is a medical condition in pregnancy characterized by a deficiency of amniotic fluid, the fluid that surrounds the fetus in the abdomen, in the amniotic sac. The limiting case is anhydramnios, where there is a complete absence of amniotic fluid. It is typically diagnosed by ultrasound when the amniotic fluid index (AFI) measures less than 5 cm or when the single deepest pocket (SDP) of amniotic fluid measures less than 2 cm. Amniotic fluid is necessary to allow for normal fetal movement, lung development, and cushioning from uterine compression. Low amniotic fluid can be attributed to a maternal, fetal, placental or idiopathic cause and can result in poor fetal outcomes including death. The prognosis of the fetus is dependent on the etiology, gestational age at diagnosis, and the severity of the oligohydramnios.

Twin-to-twin transfusion syndrome (TTTS), also known as feto-fetal transfusion syndrome (FFTS), twin oligohydramnios-polyhydramnios sequence (TOPS) and stuck twin syndrome, is a complication of monochorionic multiple pregnancies in which there is disproportionate blood supply between the fetuses. This leads to unequal levels of amniotic fluid between each fetus and usually leads to death of the undersupplied twin and, without treatment, usually death or a range of birth defects or disabilities for a surviving twin, such as underdeveloped, damaged or missing limbs, digits or organs, especially cerebral palsy.
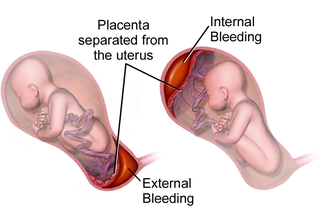
Placental abruption is when the placenta separates early from the uterus, in other words separates before childbirth. It occurs most commonly around 25 weeks of pregnancy. Symptoms may include vaginal bleeding, lower abdominal pain, and dangerously low blood pressure. Complications for the mother can include disseminated intravascular coagulopathy and kidney failure. Complications for the baby can include fetal distress, low birthweight, preterm delivery, and stillbirth.

In the fetus, the ductus venosus shunts a portion of umbilical vein blood flow directly to the inferior vena cava. Thus, it allows oxygenated blood from the placenta to bypass the liver. Compared to the 50% shunting of umbilical blood through the ductus venosus found in animal experiments, the degree of shunting in the human fetus under physiological conditions is considerably less, 30% at 20 weeks, which decreases to 18% at 32 weeks, suggesting a higher priority of the fetal liver than previously realized. In conjunction with the other fetal shunts, the foramen ovale and ductus arteriosus, it plays a critical role in preferentially shunting oxygenated blood to the fetal brain. It is a part of fetal circulation.

Inferior vena cava syndrome (IVCS) is a very rare constellation of symptoms resulting from either an obstruction, or stenosis of the inferior vena cava. It can be caused by physical invasion or compression by a pathological process or by thrombosis within the vein itself. It can also occur during pregnancy. Pregnancy leads to high venous pressure in the lower limbs, decreased blood return to the heart, decreased cardiac output due to obstruction of the inferior vena cava, sudden rise in venous pressure which can lead to placental separation, and a decrease in kidney function. All of these issues can arise from lying in the supine position during late pregnancy which can cause compression of the inferior vena cava by the uterus. Symptoms of late pregnancy inferior vena cava syndrome consist of intense pain in the right hand side, muscle twitching, hypotension, and fluid retention.
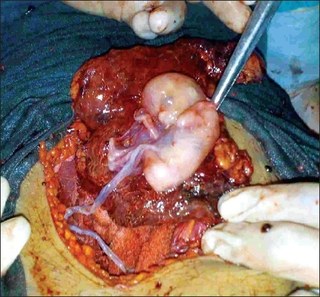
An abdominal pregnancy is a rare type of ectopic pregnancy where the embryo or fetus is growing and developing outside the uterus, in the abdomen, and not in a fallopian tube, an ovary, or the broad ligament.

Intrauterine hypoxia occurs when the fetus is deprived of an adequate supply of oxygen. It may be due to a variety of reasons such as prolapse or occlusion of the umbilical cord, placental infarction, maternal diabetes and maternal smoking. Intrauterine growth restriction may cause or be the result of hypoxia. Intrauterine hypoxia can cause cellular damage that occurs within the central nervous system. This results in an increased mortality rate, including an increased risk of sudden infant death syndrome (SIDS). Oxygen deprivation in the fetus and neonate have been implicated as either a primary or as a contributing risk factor in numerous neurological and neuropsychiatric disorders such as epilepsy, attention deficit hyperactivity disorder, eating disorders and cerebral palsy.

Placenta accreta occurs when all or part of the placenta attaches abnormally to the myometrium. Three grades of abnormal placental attachment are defined according to the depth of attachment and invasion into the muscular layers of the uterus:
- Accreta – chorionic villi attached to the myometrium, rather than being restricted within the decidua basalis.
- Increta – chorionic villi invaded into the myometrium.
- Percreta – chorionic villi invaded through the perimetrium.
Postterm pregnancy is when a woman has not yet delivered her baby after 42 weeks of gestation, two weeks beyond the typical 40-week duration of pregnancy. Postmature births carry risks for both the mother and the baby, including fetal malnutrition, meconium aspiration syndrome, and stillbirths. After the 42nd week of gestation, the placenta, which supplies the baby with nutrients and oxygen from the mother, starts aging and will eventually fail. Postterm pregnancy is a reason to induce labor.
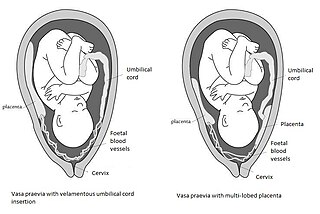
Vasa praevia is a condition in which fetal blood vessels cross or run near the internal opening of the uterus. These vessels are at risk of rupture when the supporting membranes rupture, as they are unsupported by the umbilical cord or placental tissue.

Percutaneous umbilical cord blood sampling (PUBS), also called cordocentesis, fetal blood sampling, or umbilical vein sampling is a diagnostic genetic test that examines blood from the fetal umbilical cord to detect fetal abnormalities. Fetal and maternal blood supply are typically connected in utero with one vein and two arteries to the fetus. The umbilical vein is responsible for delivering oxygen rich blood to the fetus from the mother; the umbilical arteries are responsible for removing oxygen poor blood from the fetus. This allows for the fetus’ tissues to properly perfuse. PUBS provides a means of rapid chromosome analysis and is useful when information cannot be obtained through amniocentesis, chorionic villus sampling, or ultrasound ; this test carries a significant risk of complication and is typically reserved for pregnancies determined to be at high risk for genetic defect. It has been used with mothers with immune thrombocytopenic purpura.

Velamentous cord insertion is a complication of pregnancy where the umbilical cord is inserted in the fetal membranes. It is a major cause of antepartum hemorrhage that leads to loss of fetal blood and associated with high perinatal mortality. In normal pregnancies, the umbilical cord inserts into the middle of the placental mass and is completely encased by the amniotic sac. The vessels are hence normally protected by Wharton's jelly, which prevents rupture during pregnancy and labor. In velamentous cord insertion, the vessels of the umbilical cord are improperly inserted in the chorioamniotic membrane, and hence the vessels traverse between the amnion and the chorion towards the placenta. Without Wharton's jelly protecting the vessels, the exposed vessels are susceptible to compression and rupture.

Monochorionic twins are monozygotic (identical) twins that share the same placenta. If the placenta is shared by more than two twins, these are monochorionic multiples. Monochorionic twins occur in 0.3% of all pregnancies. Seventy-five percent of monozygotic twin pregnancies are monochorionic; the remaining 25% are dichorionic diamniotic. If the placenta divides, this takes place before the third day after fertilization.
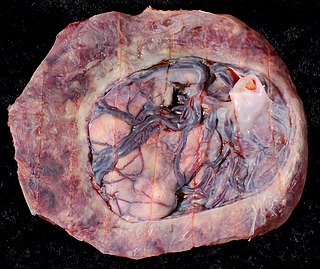
Circumvallate placenta is a rare condition affecting about 1-2% of pregnancies, in which the amnion and chorion fetal membranes essentially "double back" on the fetal side around the edges of the placenta. After delivery, a circumvallate placenta has a thick ring of membranes on its fetal surface. Circumvallate placenta is a placental morphological abnormality associated with increased fetal morbidity and mortality due to the restricted availability of nutrients and oxygen to the developing fetus.
Amnioinfusion is a method in which isotonic fluid is instilled into the uterine cavity.

Twin anemia-polycythemia sequence (TAPS) is a chronic type of unbalanced fetal transfusion in monochorionic twins that results in polycythemia in the TAPS recipient and anemia in the TAPS donor due to tiny placental anastomoses. Post-laser TAPS and spontaneous TAPS are the two forms of TAPS. Unlike twin-to-twin transfusion syndrome, which arises when twin oligohydramnios polyhydramnios sequence (TOPS) is present, TAPS develops in its absence.
The anomaly scan, also sometimes called the anatomy scan, 20-week ultrasound, or level 2 ultrasound, evaluates anatomic structures of the fetus, placenta, and maternal pelvic organs. This scan is an important and common component of routine prenatal care. The function of the ultrasound is to measure the fetus so that growth abnormalities can be recognized quickly later in pregnancy, to assess for congenital malformations and multiple pregnancies, and to plan method of delivery.
















