
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
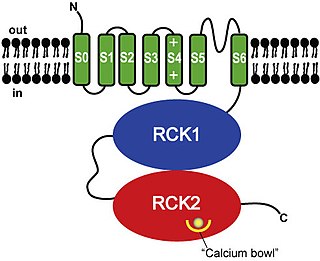
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.
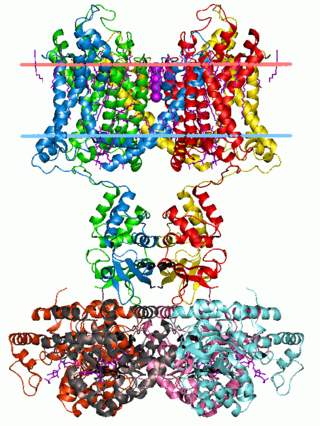
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.
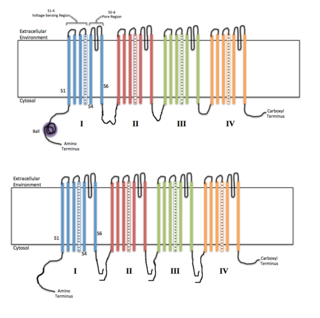
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
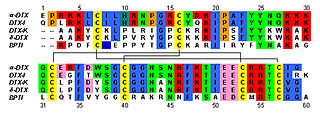
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.

Potassium voltage-gated channel subfamily A member 1 also known as Kv1.1 is a shaker related voltage-gated potassium channel that in humans is encoded by the KCNA1 gene. Isaacs syndrome is a result of an autoimmune reaction against the Kv1.1 ion channel.

Voltage-gated potassium channels (VGKCs) are transmembrane channels specific for potassium and sensitive to voltage changes in the cell's membrane potential. During action potentials, they play a crucial role in returning the depolarized cell to a resting state.

The two-pore-domain or tandem pore domain potassium channels are a family of 15 members that form what is known as leak channels which possess Goldman-Hodgkin-Katz (open) rectification. These channels are regulated by several mechanisms including signaling lipids, oxygen tension, pH, mechanical stretch, and G-proteins. Two-pore-domain potassium channels correspond structurally to a inward-rectifier potassium channel α-subunits. Each inward-rectifier potassium channel α-subunit is composed of two transmembrane α-helices, a pore helix and a potassium ion selectivity filter sequence and assembles into a tetramer forming the complete channel. The two-pore domain potassium channels instead are dimers where each subunit is essentially two α-subunits joined together.

Potassium voltage-gated channel subfamily E member 1 is a protein that in humans is encoded by the KCNE1 gene.

Calcium-activated potassium channel subunit alpha-1 also known as large conductance calcium-activated potassium channel, subfamily M, alpha member 1 (KCa1.1), or BK channel alpha subunit, is a voltage gated potassium channel encoded by the KCNMA1 gene and characterized by their large conductance of potassium ions (K+) through cell membranes.

Potassium voltage-gated channel subfamily D member 2 is a protein that in humans is encoded by the KCND2 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Potassium voltage-gated channel subfamily A member 4 also known as Kv1.4 is a protein that in humans is encoded by the KCNA4 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

Voltage-gated potassium channel subunit beta-1 is a protein that in humans is encoded by the KCNAB1 gene.
Voltage-gated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNAB2 gene.
Calcium-activated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNMB2 gene.

Calcium-activated potassium channel subunit beta-3 is a protein that in humans is encoded by the KCNMB3 gene.

Potassium voltage-gated channel subfamily S member 3 (Kv9.3) is a protein that in humans is encoded by the KCNS3 gene. KCNS3 gene belongs to the S subfamily of the potassium channel family. It is highly expressed in pulmonary artery myocytes, placenta, and parvalbumin-containing GABA neurons in brain cortex. In humans, single-nucleotide polymorphisms of the KCNS3 gene are associated with airway hyperresponsiveness, whereas decreased KCNS3 mRNA expression is found in the prefrontal cortex of patients with schizophrenia.

Calcium-activated potassium channel subunit beta-4 is a protein that in humans is encoded by the KCNMB4 gene.

Voltage-gated hydrogen channel 1 is a protein that in humans is encoded by the HVCN1 gene.
















