
Cardiac arrest, also known as sudden cardiac arrest, is when the heart suddenly and unexpectedly stops beating. As a result, blood cannot properly circulate around the body and there is diminished blood flow to the brain and other organs. When the brain does not receive enough blood, this can cause a person to lose consciousness. Coma and persistent vegetative state may result from cardiac arrest. Cardiac arrest is also identified by a lack of central pulses and abnormal or absent breathing.

An artificial cardiac pacemaker, commonly referred to as simply a pacemaker, is an implanted medical device that generates electrical pulses delivered by electrodes to one or more of the chambers of the heart. Each pulse causes the targeted chamber(s) to contract and pump blood, thus regulating the function of the electrical conduction system of the heart.

Defibrillation is a treatment for life-threatening cardiac arrhythmias, specifically ventricular fibrillation (V-Fib) and non-perfusing ventricular tachycardia (V-Tach). A defibrillator delivers a dose of electric current to the heart. Although not fully understood, this process depolarizes a large amount of the heart muscle, ending the arrhythmia. Subsequently, the body's natural pacemaker in the sinoatrial node of the heart is able to re-establish normal sinus rhythm. A heart which is in asystole (flatline) cannot be restarted by a defibrillator; it would be treated only by cardiopulmonary resuscitation (CPR) and medication, and then by cardioversion or defibrillation if it converts into a shockable rhythm.

Brugada syndrome (BrS) is a genetic disorder in which the electrical activity of the heart is abnormal due to channelopathy. It increases the risk of abnormal heart rhythms and sudden cardiac death. Those affected may have episodes of syncope. The abnormal heart rhythms seen in those with Brugada syndrome often occur at rest. They may be triggered by a fever.

Ventricular fibrillation is an abnormal heart rhythm in which the ventricles of the heart quiver. It is due to disorganized electrical activity. Ventricular fibrillation results in cardiac arrest with loss of consciousness and no pulse. This is followed by sudden cardiac death in the absence of treatment. Ventricular fibrillation is initially found in about 10% of people with cardiac arrest.

A premature ventricular contraction (PVC) is a common event where the heartbeat is initiated by Purkinje fibers in the ventricles rather than by the sinoatrial node. PVCs may cause no symptoms or may be perceived as a "skipped beat" or felt as palpitations in the chest. PVCs do not usually pose any danger.

An implantable cardioverter-defibrillator (ICD) or automated implantable cardioverter defibrillator (AICD) is a device implantable inside the body, able to perform defibrillation, and depending on the type, cardioversion and pacing of the heart. The ICD is the first-line treatment and prophylactic therapy for patients at risk for sudden cardiac death due to ventricular fibrillation and ventricular tachycardia.

Arrhythmogenic cardiomyopathy (ACM) is an inherited heart disease.
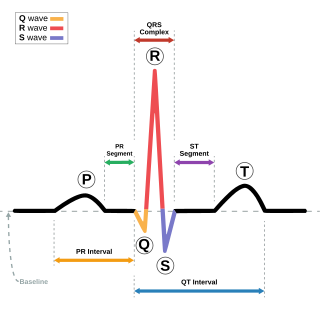
Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.

Ventricular tachycardia is a cardiovascular disorder in which fast heart rate occurs in the ventricles of the heart. Although a few seconds of VT may not result in permanent problems, longer periods are dangerous; and multiple episodes over a short period of time are referred to as an electrical storm. Short periods may occur without symptoms, or present with lightheadedness, palpitations, shortness of breath, chest pain, and decreased level of consciousness. Ventricular tachycardia may lead to coma and persistent vegetative state due to lack of blood and oxygen to the brain. Ventricular tachycardia may result in ventricular fibrillation (VF) and turn into cardiac arrest. This conversion of the VT into VF is called the degeneration of the VT. It is found initially in about 7% of people in cardiac arrest.
Multicenter Automatic Defibrillator Implantation Trial and MADIT II are implantable cardioverter defibrillator trials which investigate whether prophylactic ICD therapy in moderately high-risk coronary patients would significantly reduce death compared with patients treated with conventional therapy alone.
Clinical cardiac electrophysiology, is a branch of the medical specialty of cardiology concerned with the study and treatment of rhythm disorders of the heart. Cardiologists with expertise in this area are usually referred to as electrophysiologists. Electrophysiologists are trained in the mechanism, function, and performance of the electrical activities of the heart. Electrophysiologists work closely with other cardiologists and cardiac surgeons to assist or guide therapy for heart rhythm disturbances (arrhythmias). They are trained to perform interventional and surgical procedures to treat cardiac arrhythmia.

Cardiac resynchronisation therapy is the insertion of electrodes in the left and right ventricles of the heart, as well as on occasion the right atrium, to treat heart failure by coordinating the function of the left and right ventricles via a pacemaker, a small device inserted into the anterior chest wall.

Left atrial appendage occlusion (LAAO), also referred to as left atrial appendage closure (LAAC), is a procedure used to reduce the risk of blood clots from the left atrial appendage entering the bloodstream and causing a stroke in those with non-valvular atrial fibrillation.
Management of heart failure requires a multimodal approach. It involves a combination of lifestyle modifications, medications, and possibly the use of devices or surgery.
A wearable cardioverter defibrillator (WCD) is a non-invasive, external device for patients at risk of sudden cardiac arrest (SCA). It allows physicians time to assess their patient's arrhythmic risk and see if their ejection fraction improves before determining the next steps in patient care. It is a leased device. A summary of the device, its technology and indications was published in 2017 and reviewed by the EHRA Scientific Documents Committee.
Cameron Health was a medical device developer based in San Clemente, California, USA. Cameron Health had its European office, Cameron Health BV, in Arnhem, The Netherlands. The privately held company's focus was on a new generation of minimally invasive implantable cardioverter-defibrillator (ICD) which they called a Subcutaneous Implantable Defibrillator (S-ICD). Cameron Health's approach avoided implanting transvenous leads into the heart, which had been the usual procedure for cardiac devices. Instead, the Cameron ICD was entirely implanted outside the thoracic wall.
Cardiac contractility modulation is a therapy which is intended for the treatment of patients with moderate to severe heart failure with symptoms despite optimal medical therapy who can benefit from an improvement in cardiac output. The short- and long-term use of this therapy enhances the strength of ventricular contraction and therefore the heart's pumping capacity by modulating (adjusting) the myocardial contractility. This is provided by a pacemaker-like device that applies non-excitatory electrical signals adjusted to and synchronized with the electrical action in the cardiac cycle.
Defibrillation threshold indicates the minimum amount of energy needed to return normal rhythm to a heart that is beating in a cardiac dysrhythmia. Typical examples are the minimum amount of energy, expressed in joules, delivered by external defibrillator paddles or pads, required to break atrial fibrillation and restore normal sinus rhythm. Other common scenarios are restoring normal rhythm from atrial flutter, ventricular tachycardia or ventricular fibrillation. The defibrillation threshold ranking in these settings, from lowest to highest, would be, in order, ventricular tachycardia, atrial flutter, atrial fibrillation, ventricular fibrillation. The highest amount of energy that an external defibrillator can deliver at the present time is 360 joules biphasic. In clinical practice, the real threshold can be approximated but not exactly established, since the defibrillating shock can be delivered only once. Aside from that, energy isn't directly related to stimulus strength and efficiency, which is primarily determined by the delivered charge over time in mC and not power over time or energy, which are still used due to historical reasons. Charge based thresholds are more realistic parameters for shock efficacy. Usual values delivered by biphasic defibrillators lay between 50 and 300 mC. The amount of charge needed is influenced by bertain medications, in particular sotalol, tend to lower such threshold, while others, such as amiodarone, may increase it.

Subcutaneous implantable cardioverter defibrillator, or S-ICD, is an implantable medical device for detecting and terminating ventricular tachycardia and ventricular fibrillation in patients at risk of sudden cardiac arrest. It is a type of implantable cardioverter defibrillator but unlike the transvenous ICD, the S-ICD lead is placed just under the skin, leaving the heart and veins untouched.












