Coronary circulation is the circulation of blood in the arteries and veins that supply the heart muscle (myocardium). Coronary arteries supply oxygenated blood to the heart muscle. Cardiac veins then drain away the blood after it has been deoxygenated. Because the rest of the body, and most especially the brain, needs a steady supply of oxygenated blood that is free of all but the slightest interruptions, the heart is required to function continuously. Therefore its circulation is of major importance not only to its own tissues but to the entire body and even the level of consciousness of the brain from moment to moment. Interruptions of coronary circulation quickly cause heart attacks, in which the heart muscle is damaged by oxygen starvation. Such interruptions are usually caused by coronary ischemia linked to coronary artery disease, and sometimes to embolism from other causes like obstruction in blood flow through vessels.

Ventricular fibrillation is an abnormal heart rhythm in which the ventricles of the heart quiver. It is due to disorganized electrical activity. Ventricular fibrillation results in cardiac arrest with loss of consciousness and no pulse. This is followed by sudden cardiac death in the absence of treatment. Ventricular fibrillation is initially found in about 10% of people with cardiac arrest.

Coronary artery bypass surgery, also known as coronary artery bypass graft, is a surgical procedure to treat coronary artery disease (CAD), the buildup of plaques in the arteries of the heart. It can relieve chest pain caused by CAD, slow the progression of CAD, and increase life expectancy. It aims to bypass narrowings in heart arteries by using arteries or veins harvested from other parts of the body, thus restoring adequate blood supply to the previously ischemic heart.
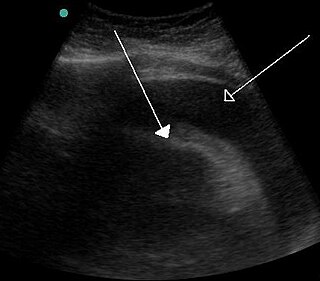
Cardiac tamponade, also known as pericardial tamponade, is a compression of the heart due to pericardial effusion. Onset may be rapid or gradual. Symptoms typically include those of obstructive shock including shortness of breath, weakness, lightheadedness, and cough. Other symptoms may relate to the underlying cause.
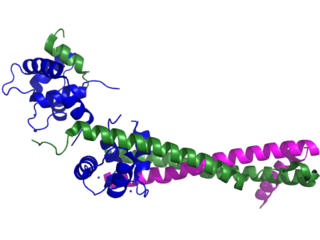
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocarditis, myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.
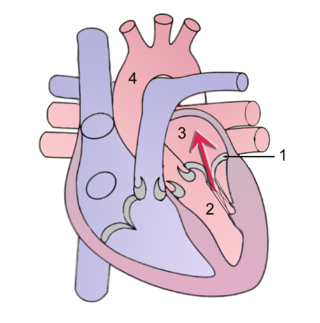
Mitral regurgitation (MR), also known as mitral insufficiency or mitral incompetence, is a form of valvular heart disease in which the mitral valve is insufficient and does not close properly when the heart pumps out blood. It is the abnormal leaking of blood backwards – regurgitation from the left ventricle, through the mitral valve, into the left atrium, when the left ventricle contracts. Mitral regurgitation is the most common form of valvular heart disease.
Dressler syndrome is a secondary form of pericarditis that occurs in the setting of injury to the heart or the pericardium. It consists of fever, pleuritic pain, pericarditis and/or pericardial effusion.
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions, most commonly pericardial effusion.

A pericardial effusion is an abnormal accumulation of fluid in the pericardial cavity. The pericardium is a two-part membrane surrounding the heart: the outer fibrous connective membrane and an inner two-layered serous membrane. The two layers of the serous membrane enclose the pericardial cavity between them. This pericardial space contains a small amount of pericardial fluid, normally 15-50 mL in volume. The pericardium, specifically the pericardial fluid provides lubrication, maintains the anatomic position of the heart in the chest (levocardia), and also serves as a barrier to protect the heart from infection and inflammation in adjacent tissues and organs.

Acute pericarditis is a type of pericarditis usually lasting less than 4 to 6 weeks. It is the most common condition affecting the pericardium.

Accelerated idioventricular rhythm is a ventricular rhythm with a rate of between 40 and 120 beats per minute. Idioventricular means “relating to or affecting the cardiac ventricle alone” and refers to any ectopic ventricular arrhythmia. Accelerated idioventricular arrhythmias are distinguished from ventricular rhythms with rates less than 40 and those faster than 120. Though some other references limit to between 60 and 100 beats per minute. It is also referred to as AIVR and "slow ventricular tachycardia."
The following outline is provided as an overview of and topical guide to cardiology, the branch of medicine dealing with disorders of the human heart. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in cardiology are called cardiologists.
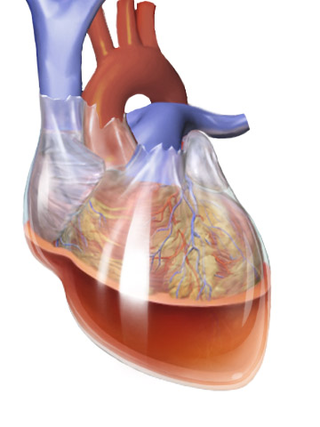
Hemopericardium refers to blood in the pericardial sac of the heart. It is clinically similar to a pericardial effusion, and, depending on the volume and rapidity with which it develops, may cause cardiac tamponade.

A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops in one of the coronary arteries of the heart, causing infarction to the heart muscle. The most common symptom is retrosternal chest pain or discomfort that classically radiates to the left shoulder, arm, or jaw. The pain may occasionally feel like heartburn. This is the dangerous type of Acute coronary syndrome.

Ventricular aneurysms are one of the many complications that may occur after a heart attack. The word aneurysm refers to a bulge or 'pocketing' of the wall or lining of a vessel commonly occurring in the blood vessels at the base of the septum, or within the aorta. In the heart, they usually arise from a patch of weakened tissue in a ventricular wall, which swells into a bubble filled with blood. This, in turn, may block the passageways leading out of the heart, leading to severely constricted blood flow to the body. Ventricular aneurysms can be fatal. They are usually non-rupturing because they are lined by scar tissue.

Reperfusion therapy is a medical treatment to restore blood flow, either through or around, blocked arteries, typically after a heart attack. Reperfusion therapy includes drugs and surgery. The drugs are thrombolytics and fibrinolytics used in a process called thrombolysis. Surgeries performed may be minimally-invasive endovascular procedures such as a percutaneous coronary intervention (PCI), which involves coronary angioplasty. The angioplasty uses the insertion of a balloon and/or stents to open up the artery. Other surgeries performed are the more invasive bypass surgeries that graft arteries around blockages.

Myocardial infarction complications may occur immediately following a myocardial infarction, or may need time to develop. After an infarction, an obvious complication is a second infarction, which may occur in the domain of another atherosclerotic coronary artery, or in the same zone if there are any live cells left in the infarct.
A diagnosis of myocardial infarction is created by integrating the history of the presenting illness and physical examination with electrocardiogram findings and cardiac markers. A coronary angiogram allows visualization of narrowings or obstructions on the heart vessels, and therapeutic measures can follow immediately. At autopsy, a pathologist can diagnose a myocardial infarction based on anatomopathological findings.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the affected area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.

Acute cardiac unloading is any maneuver, therapy, or intervention that decreases the power expenditure of the ventricle and limits the hemodynamic forces that lead to ventricular remodeling after insult or injury to the heart. This technique is being investigated as a therapeutic to aid after damage has occurred to the heart, such as after a heart attack. The theory behind this approach is that by simultaneously limiting the oxygen demand and maximizing oxygen delivery to the heart after damage has occurred, the heart is more fully able to recover. This is primarily achieved by using temporary minimally invasive mechanical circulatory support to supplant the pumping of blood by the heart. Using mechanical support decreases the workload of the heart, or unloads it.