| Hemothorax | |
|---|---|
| Other names | Haemothorax Hæmothorax Haemorrhagic pleural effusion |
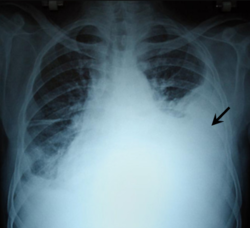 | |
| Chest X-ray showing left sided hemothorax (arrowed) | |
| Specialty | Pulmonology |
| Symptoms | Chest pain Difficulty breathing |
| Complications | Empyema Fibrothorax |
| Types | Traumatic Spontaneous |
| Causes | Trauma Cancer Endometriosis |
| Diagnostic method | Chest X-ray Ultrasound CT scan MRI Thoracentesis |
| Treatment | Tube thoracostomy Thoracotomy Fibrinolytic therapy |
| Medication | Streptokinase Urokinase |
| Prognosis | Favorable with treatment |
| Frequency | 300,000 cases in the US per year |
A hemothorax (derived from hemo- [blood] + thorax [chest], plural hemothoraces) is an accumulation of blood within the pleural cavity. The symptoms of a hemothorax may include chest pain and difficulty breathing, while the clinical signs may include reduced breath sounds on the affected side and a rapid heart rate. Hemothoraces are usually caused by an injury, but they may occur spontaneously due to cancer invading the pleural cavity, as a result of a blood clotting disorder, as an unusual manifestation of endometriosis, in response to pneumothorax, or rarely in association with other conditions.
Contents
- Background
- Signs and symptoms
- Causes
- Traumatic
- Iatrogenic
- Nontraumatic
- Mechanism
- Diagnosis
- Chest X-ray
- Other methods
- Thoracentesis
- Treatment
- Thoracostomy
- Surgery
- Other
- Prognosis
- Complications
- Epidemiology
- Other animals
- Horses
- References
- External links
Hemothoraces are usually diagnosed using a chest X-ray, but they can be identified using other forms of imaging including ultrasound, a CT scan, or an MRI. They can be differentiated from other forms of fluid within the pleural cavity by analysing a sample of the fluid, and are defined as having a hematocrit of greater than 50% that of the person's blood. Hemothoraces may be treated by draining the blood using a chest tube. Surgery may be required if the bleeding continues. If treated, the prognosis is usually good. Complications of a hemothorax include infection within the pleural cavity and the formation of scar tissue.










