Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin. While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.

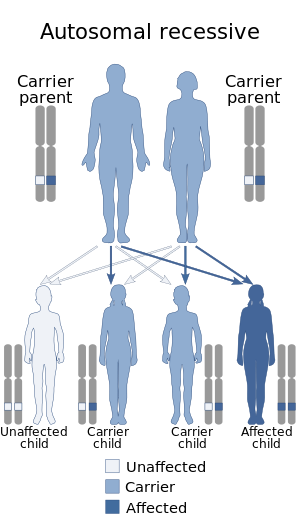
In medicine (hematology), bleeding diathesis is an unusual susceptibility to bleed (hemorrhage) mostly due to hypocoagulability, in turn caused by a coagulopathy. Therefore, this may result in the reduction of platelets being produced and leads to excessive bleeding. Several types of coagulopathy are distinguished, ranging from mild to lethal. Coagulopathy can be caused by thinning of the skin, such that the skin is weakened and is bruised easily and frequently without any trauma or injury to the body. Also, coagulopathy can be contributed by impaired wound healing or impaired clot formation.

Protein S deficiency is a disorder associated with increased risk of venous thrombosis. Protein S, a vitamin K-dependent physiological anticoagulant, acts as a nonenzymatic cofactor to activate protein C in the degradation of factor Va and factor VIIIa.
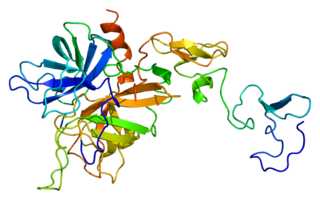
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIX, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
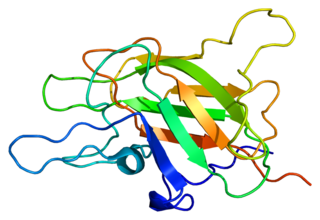
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Fresh frozen plasma (FFP) is a blood product made from the liquid portion of whole blood. It is used to treat conditions in which there are low blood clotting factors or low levels of other blood proteins. It may also be used as the replacement fluid in plasma exchange. Using ABO compatible plasma, while not required, may be recommended. Use as a volume expander is not recommended. It is given by slow injection into a vein.

Renal vein thrombosis (RVT) is the formation of a clot in the vein that drains blood from the kidneys, ultimately leading to a reduction in the drainage of one or both kidneys and the possible migration of the clot to other parts of the body. First described by German pathologist Friedrich Daniel von Recklinghausen in 1861, RVT most commonly affects two subpopulations: newly born infants with blood clotting abnormalities or dehydration and adults with nephrotic syndrome.

Warfarin-induced skin necrosis is a condition in which skin and subcutaneous tissue necrosis occurs due to acquired protein C deficiency following treatment with anti-vitamin K anticoagulants.

Protein C deficiency is a rare genetic trait that predisposes to thrombotic disease. It was first described in 1981. The disease belongs to a group of genetic disorders known as thrombophilias. Protein C deficiency is associated with an increased incidence of venous thromboembolism, whereas no association with arterial thrombotic disease has been found.

Factor X deficiency is a bleeding disorder characterized by a lack in the production of factor X (FX), an enzyme protein that causes blood to clot in the coagulation cascade. Produced in the liver FX when activated cleaves prothrombin to generate thrombin in the intrinsic pathway of coagulation. This process is vitamin K dependent and enhanced by activated factor V.

Factor VII deficiency is a bleeding disorder characterized by a lack in the production of Factor VII (FVII) (proconvertin), a protein that causes blood to clot in the coagulation cascade. After a trauma factor VII initiates the process of coagulation in conjunction with tissue factor in the extrinsic pathway.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a derivative of coumarin. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.
Prothrombin complex concentrate (PCC), also known as factor IX complex, sold under the brand name Kcentra among others, is a combination medication made up of blood clotting factors II, IX, and X. Some versions also contain factor VII. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available. It may also be used for reversal of warfarin therapy. It is given by slow injection into a vein.
Purpura fulminans is an acute, often fatal, thrombotic disorder which manifests as blood spots, bruising and discolouration of the skin resulting from coagulation in small blood vessels within the skin and rapidly leads to skin necrosis and disseminated intravascular coagulation.

Vitamin K reactions are adverse side effects that may occur after injection with vitamin K. The liver utilizes vitamin K to produce coagulation factors that help the body form blood clots which prevent excessive bleeding. Vitamin K injections are administered to newborns as a preventative measure to reduce the risk of hemorrhagic disease of the newborn (HDN).