
Retinitis pigmentosa (RP) is a genetic disorder of the eyes that causes loss of vision. Symptoms include trouble seeing at night and decreasing peripheral vision. As peripheral vision worsens, people may experience "tunnel vision". Complete blindness is uncommon. Onset of symptoms is generally gradual and often begins in childhood.

Macular degeneration, also known as age-related macular degeneration, is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, some people experience a gradual worsening of vision that may affect one or both eyes. While it does not result in complete blindness, loss of central vision can make it hard to recognize faces, drive, read, or perform other activities of daily life. Visual hallucinations may also occur.
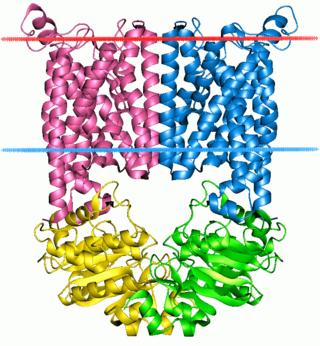
The ABC transporters, ATP synthase (ATP)-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene families. It is represented in all extant phyla, from prokaryotes to humans. ABC transporters belong to translocases.

Lipofuscin is the name given to fine yellow-brown pigment granules composed of lipid-containing residues of lysosomal digestion. It is considered to be one of the aging or "wear-and-tear" pigments, found in the liver, kidney, heart muscle, retina, adrenals, nerve cells, and ganglion cells.

Vitelliform macular dystrophy is an irregular autosomal dominant eye disorder which can cause progressive vision loss. This disorder affects the retina, specifically cells in a small area near the center of the retina called the macula. The macula is responsible for sharp central vision, which is needed for detailed tasks such as reading, driving, and recognizing faces. The condition is characterized by yellow, slightly elevated, round structures similar to the yolk of an egg.
Stargardt disease is the most common inherited single-gene retinal disease. In terms of the first description of the disease, it follows an autosomal recessive inheritance pattern, which has been later linked to bi-allelic ABCA4 gene variants (STGD1). However, there are Stargardt-like diseases with mimicking phenotypes that are referred to as STGD3 and STGD4, and have a autosomal dominant inheritance due to defects with ELOVL4 or PROM1 genes, respectively. It is characterized by macular degeneration that begins in childhood, adolescence or adulthood, resulting in progressive loss of vision.

ATP-binding cassette super-family G member 2 is a protein that in humans is encoded by the ABCG2 gene. ABCG2 has also been designated as CDw338. ABCG2 is a translocation protein used to actively pump drugs and other compounds against their concentration gradient using the bonding and hydrolysis of ATP as the energy source.

Peripherin-2 is a protein, that in humans is encoded by the PRPH2 gene. Peripherin-2 is found in the rod and cone cells of the retina of the eye. Defects in this protein result in one form of retinitis pigmentosa, an incurable blindness.

Bestrophin-1 (Best1) is a protein that, in humans, is encoded by the BEST1 gene.

Cone-rod homeobox protein is a protein that in humans is encoded by the CRX gene.

Retinaldehyde-binding protein 1 (RLBP1) also known as cellular retinaldehyde-binding protein (CRALBP) is a 36-kD water-soluble protein that in humans is encoded by the RLBP1 gene.

Rod cGMP-specific 3',5'-cyclic phosphodiesterase subunit beta is the beta subunit of the protein complex PDE6 that is encoded by the PDE6B gene. PDE6 is crucial in transmission and amplification of visual signal. The existence of this beta subunit is essential for normal PDE6 functioning. Mutations in this subunit are responsible for retinal degeneration such as retinitis pigmentosa or congenital stationary night blindness.

Elongation of very long chain fatty acids protein 4 is a protein that in humans is encoded by the ELOVL4 gene.

Cyclic nucleotide gated channel beta 3, also known as CNGB3, is a human gene encoding an ion channel protein.

Hemicentin-1 is a protein that in humans is encoded by the HMCN1 gene.

Putative ATP-binding cassette transporter sub-family C member 13 is a protein that is not present in humans. In humans, ABCC13 is a pseudogene.
Gene therapy using lentiviral vectors was being explored in early stage trials as of 2009.
Retinal gene therapy holds a promise in treating different forms of non-inherited and inherited blindness.

ATP-binding cassette, sub-family A (ABC1), member 5 is a protein that in humans is encoded by the ABCA5 gene.
Occult macular dystrophy (OMD) is a rare inherited degradation of the retina, characterized by progressive loss of function in the most sensitive part of the central retina (macula), the location of the highest concentration of light-sensitive cells (photoreceptors) but presenting no visible abnormality. "Occult" refers to the degradation in the fundus being difficult to discern. The disorder is called "dystrophy" instead of "degradation" to distinguish its genetic origin from other causes, such as age. OMD was first reported by Y. Miyake et al. in 1989.




















