Related Research Articles
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.
Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only radon-222 has a sufficiently long half-life for it to be released from the soil and rock where it is generated. Radon isotopes are the immediate decay products of radium isotopes. The instability of radon-222, its most stable isotope, makes radon one of the rarest elements. Radon will be present on Earth for several billion more years, despite its short half-life, because it is constantly being produced as a step in the decay chain of uranium-238, and that of thorium-232, each of which is an extremely abundant radioactive nuclide with a half-life of several billion years. The decay of radon produces many other short-lived nuclides, known as "radon daughters", ending at stable isotopes of lead. Radon-222 occurs in significant quantities as a step in the normal radioactive decay chain of uranium-238, also known as the uranium series, which slowly decays into a variety of radioactive nuclides and eventually decays into lead-206, which is stable. Radon-220 occurs in minute quantities as an intermediate step in the decay chain of thorium-232, also known as the thorium series, which eventually decays into lead-208, which is stable.
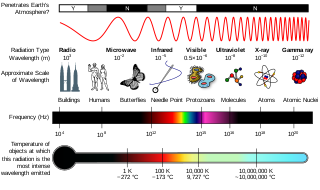
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

Radiation dosimetry in the fields of health physics and radiation protection is the measurement, calculation and assessment of the ionizing radiation dose absorbed by an object, usually the human body. This applies both internally, due to ingested or inhaled radioactive substances, or externally due to irradiation by sources of radiation.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.

Health physics, also referred to as the science of radiation protection, is the profession devoted to protecting people and their environment from potential radiation hazards, while making it possible to enjoy the beneficial uses of radiation. Health physicists normally require a four-year bachelor’s degree and qualifying experience that demonstrates a professional knowledge of the theory and application of radiation protection principles and closely related sciences. Health physicists principally work at facilities where radionuclides or other sources of ionizing radiation are used or produced; these include research, industry, education, medical facilities, nuclear power, military, environmental protection, enforcement of government regulations, and decontamination and decommissioning—the combination of education and experience for health physicists depends on the specific field in which the health physicist is engaged.

Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases, where their presence is unintended or undesirable.

The linear no-threshold model (LNT) is a dose-response model used in radiation protection to estimate stochastic health effects such as radiation-induced cancer, genetic mutations and teratogenic effects on the human body due to exposure to ionizing radiation. The model assumes a linear relationship between dose and health effects, even for very low doses where biological effects are more difficult to observe. The LNT model implies that all exposure to ionizing radiation is harmful, regardless of how low the dose is, and that the effect is cumulative over lifetime.

Radiation hormesis is the hypothesis that low doses of ionizing radiation are beneficial, stimulating the activation of repair mechanisms that protect against disease, that are not activated in absence of ionizing radiation. The reserve repair mechanisms are hypothesized to be sufficiently effective when stimulated as to not only cancel the detrimental effects of ionizing radiation but also inhibit disease not related to radiation exposure. It has been a mainstream concept since at least 2009.
The National Radiological Protection Board (NRPB) was a public authority in the UK created by the Radiological Protection Act 1970. Its statutory functions were to conduct research on radiological protection and provide advice and information on the subject to Government Departments and others. It was also authorized to provide technical services and charge for them. Originally NRPB dealt only with ionizing radiation, but its functions were extended in 1974 to non-ionizing radiation.

Lead shielding refers to the use of lead as a form of radiation protection to shield people or objects from radiation so as to reduce the effective dose. Lead can effectively attenuate certain kinds of radiation because of its high density and high atomic number; principally, it is effective at stopping gamma rays and x-rays.
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its recommendations form the basis of radiological protection policy, regulations, guidelines and practice worldwide.

Radiation dose reconstruction refers to the process of estimating radiation doses that were received by individuals or populations in the past as a result of particular exposure situations of concern. The basic principle of radiation dose reconstruction is to characterize the radiation environment to which individuals have been exposed using available information. In cases where radiation exposures can not be fully characterized based on available data, default values based on reasonable scientific assumptions can be used as substitutes. The extent to which the default values are used depends on the purpose of the reconstruction(s) being undertaken.
The health effects of radon are harmful, and include an increased chance of lung cancer. Radon is a radioactive, colorless, odorless, tasteless noble gas, which has been studied by a number of scientific and medical bodies for its effects on health. A naturally-occurring gas formed as a decay product of radium, radon is one of the densest substances that remains a gas under normal conditions, and is considered to be a health hazard due to its radioactivity. Its most stable isotope, radon-222, has a half-life of 3.8 days. Due to its high radioactivity, it has been less well studied by chemists, but a few compounds are known.
The Committee on Medical Aspects of Radiation in the Environment (COMARE) is a UK-wide advisory committee set up by the British government. It was established in 1985.
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation. Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no evidence of this has been observed.
G. William Morgan, also known as George William Morgan, health physicist and founding member of the Health Physics Society. Morgan held key health physics positions at Oak Ridge National Laboratory, the Manhattan Project and the Atomic Energy Commission. Morgan was instrumental in developing the regulations that we know today as I0 CFR 20, the Standards for Protection against Radiation.
Radiation exposure is a measure of the ionization of air due to ionizing radiation from photons. It is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air. As of 2007, "medical radiation exposure" was defined by the International Commission on Radiological Protection as exposure incurred by people as part of their own medical or dental diagnosis or treatment; by persons, other than those occupationally exposed, knowingly, while voluntarily helping in the support and comfort of patients; and by volunteers in a programme of biomedical research involving their exposure. Common medical tests and treatments involving radiation include X-rays, CT scans, mammography, lung ventilation and perfusion scans, bone scans, cardiac perfusion scan, angiography, radiation therapy, and more. Each type of test carries its own amount of radiation exposure. There are two general categories of adverse health effects caused by radiation exposure: deterministic effects and stochastic effects. Deterministic effects are due to the killing/malfunction of cells following high doses; and stochastic effects involve either cancer development in exposed individuals caused by mutation of somatic cells, or heritable disease in their offspring from mutation of reproductive (germ) cells.

Flight-time equivalent dose (FED) is an informal unit of measurement of ionizing radiation exposure. Expressed in units of flight-time, one unit of flight-time is approximately equivalent to the radiological dose received during the same unit of time spent in an airliner at cruising altitude. FED is intended as a general educational unit to enable a better understanding of radiological dose by converting dose typically presented in sieverts into units of time. FED is only meant as an educational exercise and is not a formally adopted dose measurement.

The history of radiation protection begins at the turn of the 19th and 20th centuries with the realization that ionizing radiation from natural and artificial sources can have harmful effects on living organisms. As a result, the study of radiation damage also became a part of this history.
