Related Research Articles

The sievert is a unit in the International System of Units (SI) intended to represent the stochastic health risk of ionizing radiation, which is defined as the probability of causing radiation-induced cancer and genetic damage. The sievert is important in dosimetry and radiation protection. It is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dose measurement and research into the biological effects of radiation.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.
The gray is the unit of ionizing radiation dose in the International System of Units (SI), defined as the absorption of one joule of radiation energy per kilogram of matter.

Radiation dosimetry in the fields of health physics and radiation protection is the measurement, calculation and assessment of the ionizing radiation dose absorbed by an object, usually the human body. This applies both internally, due to ingested or inhaled radioactive substances, or externally due to irradiation by sources of radiation.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.
Equivalent dose is a dose quantity H representing the stochastic health effects of low levels of ionizing radiation on the human body which represents the probability of radiation-induced cancer and genetic damage. It is derived from the physical quantity absorbed dose, but also takes into account the biological effectiveness of the radiation, which is dependent on the radiation type and energy. In the SI system of units, the unit of measure is the sievert (Sv).

Health physics, also referred to as the science of radiation protection, is the profession devoted to protecting people and their environment from potential radiation hazards, while making it possible to enjoy the beneficial uses of radiation. Health physicists normally require a four-year bachelor’s degree and qualifying experience that demonstrates a professional knowledge of the theory and application of radiation protection principles and closely related sciences. Health physicists principally work at facilities where radionuclides or other sources of ionizing radiation are used or produced; these include research, industry, education, medical facilities, nuclear power, military, environmental protection, enforcement of government regulations, and decontamination and decommissioning—the combination of education and experience for health physicists depends on the specific field in which the health physicist is engaged.
The roentgen equivalent man (rem) is a CGS unit of equivalent dose, effective dose, and committed dose, which are dose measures used to estimate potential health effects of low levels of ionizing radiation on the human body.
Absorbed dose is a dose quantity which is the measure of the energy deposited in matter by ionizing radiation per unit mass. Absorbed dose is used in the calculation of dose uptake in living tissue in both radiation protection, and radiology. It is also used to directly compare the effect of radiation on inanimate matter such as in radiation hardening.

Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases, where their presence is unintended or undesirable.

Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.

A hot particle is a microscopic piece of radioactive material that can become lodged in living tissue and deliver a concentrated dose of radiation to a small area. A generally accepted theory proposes that hot particles within the body are vastly more dangerous than external emitters delivering the same dose of radiation in a diffused manner. Other researchers claim that there is little or no difference in risk between internal and external emitters, maintaining that individuals will likely continue to accumulate radiation dose from internal sources even after being removed from the original hazard and properly decontaminated, regardless of the relative danger from an internally sourced radiation dose compared to an equivalent externally sourced radiation dose.
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its recommendations form the basis of radiological protection policy, regulations, guidelines and practice worldwide.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the effects of ionizing radiation on living things, in particular health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.
In radiobiology, the relative biological effectiveness is the ratio of biological effectiveness of one type of ionizing radiation relative to another, given the same amount of absorbed energy. The RBE is an empirical value that varies depending on the type of ionizing radiation, the energies involved, the biological effects being considered such as cell death, and the oxygen tension of the tissues or so-called oxygen effect.
Committed dose equivalent and Committed effective dose equivalent are dose quantities used in the United States system of radiological protection for irradiation due to an internal source.
The Deep-dose equivalent (DDE) is a measure of external radiation exposure defined by US regulations. It is reported alongside eye and shallow dose equivalents on typical US dosimetry reports. It represents the dose equivalent at a tissue depth of 1 cm (1000 mg/cm2) due to external whole-body exposure to ionizing radiation.
Internal dosimetry is the science and art of internal ionising radiation dose assessment due to radionuclides incorporated inside the human body.
Effective dose is a dose quantity in the International Commission on Radiological Protection (ICRP) system of radiological protection.
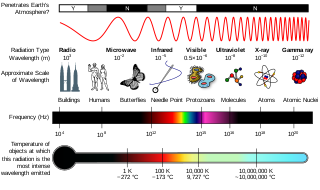
Radiation exposure is a measure of the ionization of air due to ionizing radiation from photons. It is defined as the electric charge freed by such radiation in a specified volume of air divided by the mass of that air. As of 2007, "medical radiation exposure" was defined by the International Commission on Radiological Protection as exposure incurred by people as part of their own medical or dental diagnosis or treatment; by persons, other than those occupationally exposed, knowingly, while voluntarily helping in the support and comfort of patients; and by volunteers in a programme of biomedical research involving their exposure. Common medical tests and treatments involving radiation include X-rays, CT scans, mammography, lung ventilation and perfusion scans, bone scans, cardiac perfusion scan, angiography, radiation therapy, and more. Each type of test carries its own amount of radiation exposure. There are two general categories of adverse health effects caused by radiation exposure: deterministic effects and stochastic effects. Deterministic effects are due to the killing/malfunction of cells following high doses; and stochastic effects involve either cancer development in exposed individuals caused by mutation of somatic cells, or heritable disease in their offspring from mutation of reproductive (germ) cells.
References
- ↑ ICRP publication 103 - Paragraph 83.
- ↑ ICRP Publication 103 paragraph 140
- 1 2 ICRP publication 103 - Glossary.
- ↑ ICRP publication 103 - paragraph B225 and glossary.
- ↑ ICRP publication 103 - Paragraph 144.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2015-09-24. Retrieved 2014-10-31.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ ICPR: Draft report for consultation Archived 2015-06-22 at the Wayback Machine
- ↑ Icrp (2007). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Vol. 37. ISBN 978-0-7020-3048-2. Archived from the original on 16 November 2012. Retrieved 17 May 2012.
{{cite book}}:|journal=ignored (help) - ↑ Blears, Hazel (4 March 2003). "Written answers: Radiation". Hansard .
ECRR is not a formal scientific advisory committee to the European Commission or to the European Parliament
- ↑ European Committee on Radiation Risk (2010). Busby, Chris; et al. (eds.). 2010 recommendations of the ECRR : the health effects of exposure to low doses of ionizing radiation (PDF) (Regulators' ed.). Aberystwyth: Green Audit. ISBN 978-1-897761-16-8. Archived from the original (PDF) on 21 July 2012. Retrieved 18 May 2012.
- ↑ The Response of the National Radiological Protection Board to the Report of the Committee Examining Radiation Risks of Internal Emitters (CERRIE), HPA, UK, 2005
- ↑ Rivkees, Scott A.; Sklar, Charles; Freemark, Michael (1998). "The Management of Graves' Disease in Children, with Special Emphasis on Radioiodine Treatment". Journal of Clinical Endocrinology & Metabolism. 83 (11): 3767–76. doi: 10.1210/jcem.83.11.5239 . PMID 9814445.
- ↑ Rowland, R.E. (1994). Radium in Humans: A Review of U.S. Studies (PDF). Argonne National Laboratory. Retrieved 24 May 2012.
- ↑ Wynn, Volkert; Hoffman, Timothy (1999). "Therapeutic Radiopharmaceuticals afrtin=2+3=9000" (PDF). Chemical Reviews. 99 (9): 2269–92. doi:10.1021/cr9804386. PMID 11749482.
- ↑ NRC Glossary
- ↑ "The confusing world of radiation dosimetry" - M.A. Boyd, Waste Management conference paper 2009, U.S. Environmental Protection Agency. An account of differences between USA and ICRP dosimetry systems.
- US nuclear regulatory commission glossary
- Argonne national laboratory glossary
- Limitation of Exposure to Ionizing Radiation (Report No. 116). National Council on Radiation Protection and Measurements (NCRP).