Related Research Articles

Clostridium botulinum is a gram-positive, rod-shaped, anaerobic, spore-forming, motile bacterium with the ability to produce botulinum toxin, which is a neurotoxin.

Clostridium is a genus of anaerobic, Gram-positive bacteria. Species of Clostridium inhabit soils and the intestinal tract of animals, including humans. This genus includes several significant human pathogens, including the causative agents of botulism and tetanus. It also formerly included an important cause of diarrhea, Clostridioides difficile, which was reclassified into the Clostridioides genus in 2016.

Clostridium perfringens is a Gram-positive, bacillus (rod-shaped), anaerobic, spore-forming pathogenic bacterium of the genus Clostridium. C. perfringens is ever-present in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil. It has the shortest reported generation time of any organism at 6.3 minutes in thioglycolate medium.

Membrane alanyl aminopeptidase also known as alanyl aminopeptidase (AAP) or aminopeptidase N (AP-N) is an enzyme that in humans is encoded by the ANPEP gene.
Collagenases are enzymes that break the peptide bonds in collagen. They assist in destroying extracellular structures in the pathogenesis of bacteria such as Clostridium. They are considered a virulence factor, facilitating the spread of gas gangrene. They normally target the connective tissue in muscle cells and other body organs.
Microbial collagenase is an enzyme. This enzyme catalyses the following chemical reaction
Clostripain is a proteinase that cleaves proteins on the carboxyl peptide bond of arginine. It was isolated from Clostridium histolyticum. The isoelectric point of the enzyme is 4.8-4.9, and optimum pH is 7.4~7.8. The composition of the enzyme is indicated to be of two chains of relative molecular mass 45,000 and 12,500.
Clostridial necrotizing enteritis (CNE) is a severe and potentially fatal type of food poisoning caused by a β-toxin of Clostridium perfringens, Type C. It occurs in some developing regions, particularly in New Guinea, where it is known as pig-bel. The disease was also documented in Germany following World War II, where it was called Darmbrand (literally "bowel fire," or bowel necrosis). The toxin is normally inactivated by certain proteolytic enzymes and by normal cooking, but when these protections are impeded by diverse factors, and high protein is consumed, the disease can emerge.
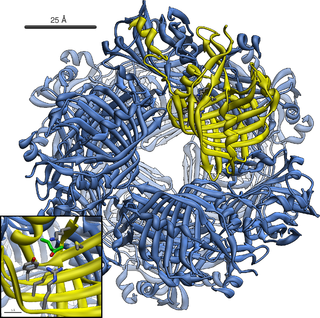
Acetoacetate decarboxylase is an enzyme involved in both the ketone body production pathway in humans and other mammals, and solventogenesis in bacteria. Acetoacetate decarboxylase plays a key role in solvent production by catalyzing the decarboxylation of acetoacetate, yielding acetone and carbon dioxide.

Xaa-Pro aminopeptidase 1 is an enzyme that in humans is encoded by the XPNPEP1 gene.
Clostridioides difficile toxin B (TcdB) is a cytotoxin produced by the bacteria Clostridioides difficile. It is one of two major kinds of toxins produced by C. difficile, the other being a related enterotoxin. Both are very potent and lethal.

The AB toxins are two-component protein complexes secreted by a number of pathogenic bacteria, though there is a pore-forming AB toxin found in the eggs of a snail. They can be classified as Type III toxins because they interfere with internal cell function. They are named AB toxins due to their components: the "A" component is usually the "active" portion, and the "B" component is usually the "binding" portion. The "A" subunit possesses enzyme activity, and is transferred to the host cell following a conformational change in the membrane-bound transport "B" subunit. These proteins consist of two independent polypeptides, which correspond to the A/B subunit moieties. The enzyme component (A) enters the cell through endosomes produced by the oligomeric binding/translocation protein (B), and prevents actin polymerisation through ADP-ribosylation of monomeric G-actin.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxins. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.

Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
Enkephalinases are enzymes that degrade endogenous enkephalin opioid peptides. They include:
Hathewaya histolytica is a species of bacteria found in feces and the soil. It is a motile, gram-positive, aerotolerant anaerobe. H. histolytica is pathogenic in many species, including guinea pigs, mice, and rabbits, and humans. H. histolytica has been shown to cause gas gangrene, often in association with other bacteria species.
Clostridium innocuum is an anaerobic, non-motile, gram-positive bacterium that reproduces by sporulation. While there are over 130 species of Clostridium, C. innocuum is the third most commonly isolated. Although it is not normally considered an aggressive human pathogen, it has been isolated in some disease processes. C. innocuum and other Clostridium line the oropharynx and gastrointestinal tract, and are considered normal gut flora.
In molecular biology, VR-RNA is a small RNA produced by Clostridium perfringens. It functions as a regulator of the two-component VirR/VirS system.
Xaa-Pro aminopeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Directed enzyme prodrug therapy (DEPT) uses enzymes artificially introduced into the body to convert prodrugs, which have no or poor biologically activity, to the active form in the desired location within the body. Many chemotherapy drugs for cancer lack tumour specificity and the doses required to reach therapeutic levels in the tumour are often toxic to other tissues. DEPT strategies are an experimental method of reducing the systemic toxicity of a drug, by achieving high levels of the active drug only at the desired site. This article describes the variations of DEPT technology.
References
- ↑ Kessler E, Yaron A (January 1973). "A novel aminopeptidase from Clostridium histolyticum". Biochemical and Biophysical Research Communications. 50 (2): 405–12. doi:10.1016/0006-291x(73)90855-3. PMID 4631895.
- ↑ Kessler E, Yaron A (March 1976). "An extracellular aminopeptidase from Clostridium histolyticum". European Journal of Biochemistry. 63 (1): 271–87. doi:10.1111/j.1432-1033.1976.tb10229.x. PMID 4318.
- ↑ Kessler E, Yaron A (1976). "Extracellular aminopeptidase from Clostridium histolyticum". Part B: Proteolytic Enzymes. Methods in Enzymology. Vol. 45. pp. 544–52. doi:10.1016/s0076-6879(76)45048-6. ISBN 978-0-12-181945-3. PMID 13266.