Hazards
Biosafety

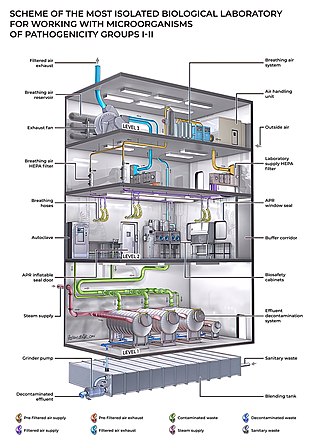
Biosafety hazards to workers from synthetic biology are similar to those in existing fields of biotechnology, mainly exposure to pathogens and toxic chemicals used in a laboratory or industrial setting. [1] [4] These include hazardous chemicals; biological hazards including organisms, prions, and biologically-derived toxins; physical hazards such as ergonomic hazards, radiation, and noise hazards; and additional hazards of injury from autoclaves, centrifuges, compressed gas, cryogens, and electrical hazards. [5]
Novel protocells or xenobiological organisms, as well as gene editing of higher animals, may have novel biosafety hazards that affect their risk assessment. As of 2018, most laboratory biosafety guidance is based on preventing exposure to existing rather than new pathogens. [4] Lentiviral vectors derived from the HIV-1 virus are widely used in gene therapy due to their unique ability to infect both dividing and non-dividing cells, but unintentional exposure of workers could lead to cancer and other diseases. [1] [4] In the case of an unintentional exposure, antiretroviral drugs can be used as post-exposure prophylaxis. [4]
Given the overlap between synthetic biology and the do-it-yourself biology movement, concerns have been raised that its practitioners may not abide by risk assessment and biosafety practices required of professionals, [2] : 39 although it has been suggested that an informal code of ethics exists that recognizes health risks and other adverse outcomes. [3] : 15
Biosecurity

The rise of synthetic biology has also spurred biosecurity concerns that synthetic or redesigned organisms could be engineered for bioterrorism. This is considered possible but unlikely given the resources needed to perform this kind of research. [1] However, synthetic biology could expand the group of people with relevant capabilities, and reduce the amount of time needed to develop them. [6] : 2–7
A 2018 National Academies of Sciences, Engineering, and Medicine (NASEM) report identified three capabilities as being of greatest concern. The first is the recreation of known pathogens from scratch, for example using genome synthesis to recreate historical viruses such as the Spanish Flu virus or polio virus. [3] : 12, 14 [6] : 2–7 Current technology allows genome synthesis for almost any mammalian virus, the sequences of known human viruses are publicly available, and the procedure has relatively low cost and requires access to basic laboratory equipment. However, the pathogens would have known properties and could be mitigated by standard public health measures, and could be partially prevented by screening of commercially produced DNA molecules. In contrast to viruses, creating existing bacteria or completely novel pathogens from scratch was not yet possible as of 2018, and was considered a low risk. [6] : 39–43, 54–56
Another capability of concern cited by NASEM is engineering existing pathogens to be more dangerous. This includes altering the targeted host or tissue, as well as enhancing the pathogen's replication, virulence, transmissibility, or stability; or its ability to produce toxins, reactivate from a dormant state, evade natural or vaccine-induced immunity, or evade detection. The NASEM considered engineered bacteria to be a higher risk than viruses because they are easier to manipulate and their genomes are more stable over time. [6] : 5, 44–53
A final capability of concern cited by NASEM is engineering microbes to produce harmful biochemicals. Metabolic engineering of microorganisms is a well established field that has targeted production of fuels, chemicals, food ingredients, and pharmaceuticals, but it could be used to produce toxins, antimetabolites, controlled substances, explosives, or chemical weapons. This was considered to be a higher risk for naturally occurring substances than for artificial ones. [6] : 59–65
There is also the possibility of novel threats that were considered lower risks by NASEM due to their technical challenges. Delivery of an engineered organism into the human microbiome has the challenges of delivery and persistence in the microbiome, though an attack would be difficult to detect and mitigate. Pathogens engineered to alter the human immune system by causing immunodeficiency, hyperreactivity, or autoimmunity, or to directly alter the human genome, were also considered lower-risk due to extreme technical challenges. [6] : 65–83
Environmental
Environmental hazards include toxicity to animals and plants, as well as adverse effects on biodiversity and ecosystem services. For example, a toxin engineered into a plant to resist specific insect pests may also affect other invertebrates. [2] : 18 Some highly speculative hazards include engineered organisms becoming invasive and outcompeting natural ones, and horizontal gene transfer from engineered to natural organisms. [7] [8] Gene drives to suppress disease vectors may inadvertently affect the target species' fitness and alter ecosystem balance. [8]
In addition, synthetic biology could lead to land-use changes, such as non-food synthetic organisms displacing other agricultural uses or wild land. It could also cause products to be produced by non-agricultural means or through large-scale commercial farming, which could economically outcompete small-scale farmers. Finally, there is a risk that conservation methods based on synthetic biology, such as de-extinction, may reduce support for traditional conservation efforts. [8] [9]