
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant as carbon dioxide, and more than 500 times as abundant as neon. Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.

The noble gases make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).

Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

Krypton difluoride, KrF2 is a chemical compound of krypton and fluorine. It was the first compound of krypton discovered. It is a volatile, colourless solid at room temperature. The structure of the KrF2 molecule is linear, with Kr−F distances of 188.9 pm. It reacts with strong Lewis acids to form salts of the KrF+ and Kr
2F+
3 cations.
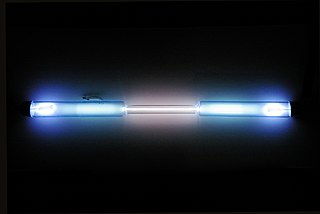
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.
A tetrafluoride is a chemical compound with four fluorines in its formula.
Xenon monochloride (XeCl) is an exciplex which is used in excimer lasers and excimer lamps emitting near ultraviolet light at 308 nm. It is most commonly used in medicine. Xenon monochloride was first synthesized in the 1960s. Its kinetic scheme is very complex and its state changes occur on a nanosecond timescale. In the gaseous state, at least two kinds of xenon monochloride are known: XeCl and Xe
2Cl, whereas complex aggregates form in the solid state in noble gas matrices. The excited state of xenon resembles halogens and it reacts with them to form excited molecular compounds.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Diborane(2) or diborene is a theoretical/hypothetical inorganic compound with the formula B2H2. Diborenes also refers to a series of molecules with a formula R:(BH)=(BH):R, where R is an organic group. B2H2 are unstable under ambient conditions. They are synthesized by pulsed laser ablation of boron in a mixed hydrogen-argon gas atmosphere. Upon cooling the mixture, the argon gas changes into a solid, thereby stabilizing the trapped diboranes.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallize with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.

Diargon or the argon dimer is a molecule containing two argon atoms. Normally, this is only very weakly bound together by van der Waals forces. However, in an excited state, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperatures, argon gas can have a few percent of diargon molecules.
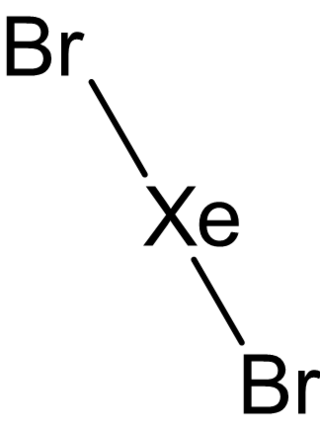
Xenon dibromide is an unstable chemical compound with the chemical formula XeBr2. It was only produced by the decomposition of iodine-129:

Superelectrophilic anions are a class of molecular ions that exhibit highly electrophilic reaction behavior despite their overall negative charge. Thus, they are even able to bind the unreactive noble gases or molecular nitrogen at room temperature. The only representatives known so far are the fragment ions of the type [B12X11]– derived from the closo-dodecaborate dianions [B12X12]2–. X represents a substituent connected to a boron atom (cf. Fig. 1). For this reason, the following article deals exclusively with superelectrophilic anions of this type.

Radon compounds are compounds formed by the element radon (Rn). Radon is a member of the zero-valence elements that are called noble gases, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas at standard conditions, unlike its decay-chain parents, it can readily be extracted from them for research.
Organokrypton chemistry describes the synthesis and properties of chemical compounds containing a carbon to krypton chemical bond.








