Related Research Articles

Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine. It is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-.

Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. Present in animals since early stages of evolution, in humans it plays roles in behavior that include social bonding, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during labour. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in maternal bonding and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.
Proprotein convertases (PPCs) are a family of proteins that activate other proteins. Many proteins are inactive when they are first synthesized, because they contain chains of amino acids that block their activity. Proprotein convertases remove those chains and activate the protein. The prototypical proprotein convertase is furin. Proprotein convertases have medical significance, because they are involved in many important biological processes, such as cholesterol synthesis. Compounds called proprotein convertase inhibitors can block their action, and block the target proteins from becoming active. Many proprotein convertases, especially furin and PACE4, are involved in pathological processes such as viral infection, inflammation, hypercholesterolemia, and cancer, and have been postulated as therapeutic targets for some of these diseases.

Glutamate carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction
In enzymology, a peptidylglycine monooxygenase (EC 1.14.17.3) is an enzyme that catalyzes the chemical reaction
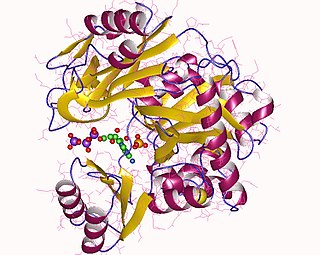
Phosphoribosylamine—glycine ligase, also known as glycinamide ribonucleotide synthetase (GARS), (EC 6.3.4.13) is an enzyme that catalyzes the chemical reaction

In enzymology, a phosphoribosylformylglycinamidine synthase (EC 6.3.5.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a [acetyl-CoA carboxylase] kinase is an enzyme that catalyzes the chemical reaction
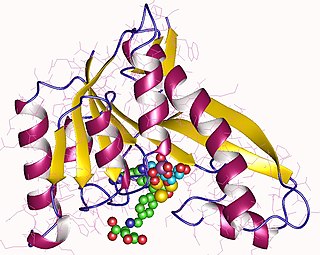
Phosphoribosylglycinamide formyltransferase (EC 2.1.2.2, 2-amino-N-ribosylacetamide 5'-phosphate transformylase, GAR formyltransferase, GAR transformylase, glycinamide ribonucleotide transformylase, GAR TFase, 5,10-methenyltetrahydrofolate:2-amino-N-ribosylacetamide ribonucleotide transformylase) is an enzyme with systematic name 10-formyltetrahydrofolate:5'-phosphoribosylglycinamide N-formyltransferase. This enzyme catalyses the following chemical reaction

Melanocyte-inhibiting factor (also known as Pro-Leu-Gly-NH2, Melanostatin, MSH release–inhibiting hormone or MIF-1) is an endogenous peptide fragment derived from cleavage of the hormone oxytocin, but having generally different actions in the body. MIF-1 produces multiple effects, both blocking the effects of opioid receptor activation, while at the same time acting as a positive allosteric modulator of the D2 and D4 dopamine receptor subtypes, as well as inhibiting release of other neuropeptides such as alpha-MSH, and potentiating melatonin activity.
Serratia marcescens nuclease is an enzyme. This enzyme catalyses the following chemical reaction
Non-stereospecific dipeptidase is an enzyme. This enzyme catalyses the following chemical reaction
Peptidyl-dipeptidase B is an enzyme. It catalyses the following chemical reaction
Pyroglutamyl-peptidase II is an enzyme. This enzyme catalyses the following chemical reaction
Stratum corneum chymotryptic enzyme is an enzyme. This enzyme catalyses the following chemical reaction
Dactylysin is an enzyme. This enzyme catalyses the following chemical reaction
Magnolysin is an enzyme. This enzyme catalyses the following chemical reaction
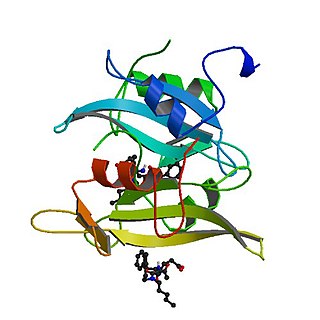
Tyrosine-protein kinase CSK also known as C-terminal Src kinase is an enzyme that, in humans, is encoded by the CSK gene. This enzyme phosphorylates tyrosine residues located in the C-terminal end of Src-family kinases (SFKs) including SRC, HCK, FYN, LCK, LYN and YES1.
Oxytocinase is a type of enzyme that metabolizes the endogenous neuropeptide, oxytocin. The most well-characterized oxytocinase is leucyl/cystinyl aminopeptidase, which is also an enkephalinase. Other oxytocinases are also known. During pregnancy, oxytocinase plays a role in balancing concentration of oxytocin by degrading the oxytocin produced by the fetus, as production of oxytocin increases with growth of fetus. One study found that concentration level of oxytocinase increased progressively with gestational age until labor, which indicates that pregnancy development can be statistically evaluated by comparing oxytocinase levels.

Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3. It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase, bacterial leucyl aminopeptidase, leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and, to a lesser extent, glutamyl aminopeptidase, as well as other aminopeptidases. It does not inhibit arginyl aminopeptidase. Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo. It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.
References
- ↑ Fruhaufová, L.; Suska-Brezezinska, E.; Barth, T.; Rychlik, I. (1973). "Rat liver enzyme inactivating oxytocin and its deamino-carba analogues". Collection of Czechoslovak Chemical Communications. 38 (9): 2793–2798. doi:10.1135/cccc19732793.
- ↑ Nardacci NJ, Mukhopadhyay S, Campbell BJ (January 1975). "Partial purification and characterization of the antidiuretic hormone-inactivating enzyme from renal plasma membranes". Biochimica et Biophysica Acta (BBA) - Enzymology. 377 (1): 146–57. doi:10.1016/0005-2744(75)90295-8. PMID 1122284.
- ↑ Simmons WH, Walter R (January 1980). "Carboxamidopeptidase: purification and characterization of a neurohypophyseal hormone inactivating peptidase from toad skin". Biochemistry. 19 (1): 39–48. doi:10.1021/bi00542a007. PMID 6766314.