 | |
| Names | |
|---|---|
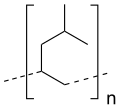
| IUPAC name Poly[1-(2-methylpropyl)ethylene] | |
| Other names Poly(4-methyl-1-pentene); PMP | |
| Identifiers | |
| ChemSpider |
|
CompTox Dashboard (EPA) | |
| Properties | |
| (C6H12)n | |
| Molar mass | Variable |
| Density | 0.833 g/mL |
| Melting point | 240 °C (464 °F; 513 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Polymethylpentene (PMP), also known as poly(4-methyl-1-pentene). It is used for gas-permeable packaging, autoclavable medical and laboratory equipment, microwave components, and cookware. It is commonly called TPX, which is a trademark of Mitsui Chemicals. [1]

