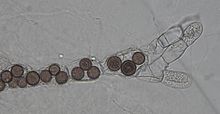
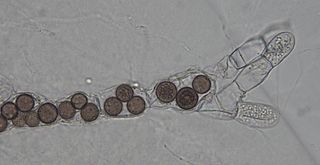
Chytridiomycota are a division of zoosporic organisms in the kingdom Fungi, informally known as chytrids. The name is derived from the Ancient Greek χυτρίδιον (khutrídion), meaning "little pot", describing the structure containing unreleased zoospores. Chytrids are one of the earliest diverging fungal lineages, and their membership in kingdom Fungi is demonstrated with chitin cell walls, a posterior whiplash flagellum, absorptive nutrition, use of glycogen as an energy storage compound, and synthesis of lysine by the α-amino adipic acid (AAA) pathway.
Hyphochytrids are eukaryotic organisms in the group of Stramenopiles (Heterokonta).

The Spitzenkörper is a structure found in fungal hyphae that is the organizing center for hyphal growth and morphogenesis. It consists of many small vesicles and is present in growing hyphal tips, during spore germination, and where branch formation occurs. Its position in the hyphal tip correlates with the direction of hyphal growth. The Spitzenkörper is a part of the endomembrane system in fungi.
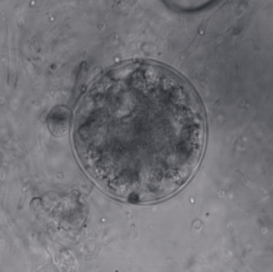
Fungi of the order Chytridiales, like other members of its division, may either have a monocentric thallus or a polycentric rhizomycelium. When the ribosomal genes of members classified in this order were first examined using molecular techniques, it was discovered that the order contained some species that were not related. With the culture and characterization of Chytridium olla, the type species of this order, the limits of the Chytridiales were established. The Chytridiales is now monophyletic and species such as Polychytrium aggregatum, Chytriomyces angularis and Cladochytrium replicatum have been transferred to other orders.

Rhizophydiales are an important group of chytrid fungi. They are found in soil as well as marine and fresh water habitats where they function as parasites and decomposers.

Spizellomycetales is an order of fungi in the Chytridiomycetes. Spizellomycetalean chytrids are essentially ubiquitous zoospore-producing fungi found in soils where they decompose pollen. Recently they have also been found in dung and harsh alpine environments, greatly expanding the range of habitats where one can expect to find these fungi.

Blastocladiomycota is one of the currently recognized phyla within the kingdom Fungi. Blastocladiomycota was originally the order Blastocladiales within the phylum Chytridiomycota until molecular and zoospore ultrastructural characters were used to demonstrate it was not monophyletic with Chytridiomycota. The order was first erected by Petersen for a single genus, Blastocladia, which was originally considered a member of the oomycetes. Accordingly, members of Blastocladiomycota are often referred to colloquially as "chytrids." However, some feel "chytrid" should refer only to members of Chytridiomycota. Thus, members of Blastocladiomyota are commonly called "blastoclads" by mycologists. Alternatively, members of Blastocladiomycota, Chytridiomycota, and Neocallimastigomycota lumped together as the zoosporic true fungi. Blastocladiomycota contains 5 families and approximately 12 genera. This early diverging branch of kingdom Fungi is the first to exhibit alternation of generations. As well, two (once) popular model organisms—Allomyces macrogynus and Blastocladiella emersonii—belong to this phylum.

Holomycota or Nucletmycea are a basal Opisthokont clade as sister of the Holozoa. It consists of the Cristidiscoidea and the kingdom Fungi. The position of nucleariids, unicellular free-living phagotrophic amoebae, as the earliest lineage of Holomycota suggests that animals and fungi independently acquired complex multicellularity from a common unicellular ancestor and that the osmotrophic lifestyle was originated later in the divergence of this eukaryotic lineage. Opisthosporidians is a recently proposed taxonomic group that includes aphelids, Microsporidia and Cryptomycota, three groups of endoparasites.
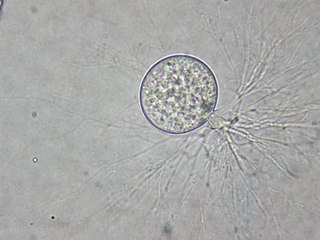
Cryptomycota , Rozellida, or Rozellomycota are a clade of micro-organisms that are either fungi or a sister group to fungi. They differ from classical fungi in that they lack chitinous cell walls at any trophic stage in their lifecycle, as reported by Jones and colleagues in 2011. Despite their unconventional feeding habits, chitin has been observed in the inner layer of resting spores, and in immature resting spores for some species of Rozella, as indicated with calcofluor-white stain as well as the presence of a fungal-specific chitin synthase gene.

Synchytrium is a large genus of plant pathogens within the phylum Chytridiomycota. Species are commonly known as false rust or wart disease. Approximately 200 species are described, and all are obligate parasites of angiosperms, ferns, or mosses. Early species were mistakenly classified among the higher fungi because of their superficial similarity to the rust fungi. Anton de Bary and Mikhail S. Woronin recognized the true nature of these fungi and established the genus to accommodate Synchytrium taraxaci, which grows on dandelions, and S. succisae, which grows on Succisa pratensis. Synchytrium taraxaci is the type of the genus. The genus has been divided into 6 subgenera based on differences in life cycles.
Olpidium is a fungal genus in the family Olpidiaceae. Members of Olpidium are zoosporic pathogens of plants, animals, fungi, and oomycetes.
Allomyces macrogynus is a species of fungus in the family Blastocladiaceae. It was first described by mycologist Ralph Emerson in 1941 as a variety of Allomyces javanicus, and later given distinct species status in 1954. Its genome has been sequenced by the Broad Institute.

Allomyces is a genus of fungi in the family Blastocladiaceae. It was circumscribed by British mycologist Edwin John Butler in 1911. Species in the genus have a polycentric thallus and reproduce sexually or asexually by zoospores that have a whiplash-like flagella. They are mostly isolated from soils in tropical countries, commonly in ponds, rice fields, and slow-moving rivers.

Physoderma is a genus of chytrid fungi. Described by German botanist Karl Friedrich Wilhelm Wallroth in 1833, the genus contains some species that are parasitic on vascular plants, including P. alfalfae and P. maydis, causative agents of crown wart of alfalfa and brown spot of corn, respectively. Of the chytrid genera, Physoderma is the oldest. However, species were confused with the rust fungi, the genus Synchytrium, and the genus Protomyces of Ascomycota. Members of Physoderma are obligate parasites of pteridophytes and angiosperms. There are approximately 80 species within this genus.

Chytriomyces is the type genus of fungi in the family Chytriomycetaceae. The genus was described by mycologist John Sidney Karling in 1945. The family, created by Peter Letcher in 2011, contains species with a Group I-type zoospore, distinguishing it from Chytridiaceae members, which have a Group II-type zoospore.
Allomyces anomalus is a species of fungus. A common water mold found throughout Asia and Africa, it is a host of the endoparasite Rozella allomycis.
Cladochytriales is an order of chytrid fungi. It is the only order in the monotypic class Cladochytriomycetes. The order was described in 2009 to accommodate a monophyletic clade containing many genera of chytrid fungi often observed growing over decaying plant tissue and other cellulosic substrates from aquatic habitats and humid soils.
Aphelidium species are endoparasites of freshwater green algae. Aphelidium belongs to the phylum Aphelida, and is part of the Opisthosporidia, a sister clade to Fungi. The cells of Aphelidium are much smaller than the cells of its green algae host, which is protected by a robust cell wall. Aphelidium have evolved a remarkable life cycle to defeat host's defenses.

Physodermatacae is a family of chytrid fungi in the order Physodermatales. Species in the family have a parasitic relationship with the host's physoderma. This family is distinctive in that it contains a thick wall around the sporangia to resist against unfavorable conditions. Sporangia releases from a host plant when rotting, dispersal is carried through the air. This family is not to be confused or related to basidiomycetes rusts and smut fungi. This parasite is distributed all across the world in aquatic, semi aquatic wetlands and in some ferns.
Marilyn Rose Noyes Mollicone was an American botanist advancing mycology in Maine and advocating for naturalist education.