Related Research Articles
An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to drug preparation, route of administration, site-specific targeting, metabolism, and toxicity are used to optimize efficacy and safety, and to improve patient convenience and compliance. Drug delivery is aimed at altering a drug's pharmacokinetics and specificity by formulating it with different excipients, drug carriers, and medical devices. There is additional emphasis on increasing the bioavailability and duration of action of a drug to improve therapeutic outcomes. Some research has also been focused on improving safety for the person administering the medication. For example, several types of microneedle patches have been developed for administering vaccines and other medications to reduce the risk of needlestick injury.

Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and imaging. Mesoporous ordered silica films have been also obtained with different pore topologies.
Thiolated polymers – designated thiomers – are functional polymers used in biotechnology product development with the intention to prolong mucosal drug residence time and to enhance absorption of drugs. The name thiomer was coined by Andreas Bernkop-Schnürch in 2000. Thiomers have thiol bearing side chains. Sulfhydryl ligands of low molecular mass are covalently bound to a polymeric backbone consisting of mainly biodegradable polymers, such as chitosan, hyaluronic acid, cellulose derivatives, pullulan, starch, gelatin, polyacrylates, cyclodextrins, or silicones.
In the pharmaceutical industry, drug dissolution testing is routinely used to provide critical in vitro drug release information for both quality control purposes, i.e., to assess batch-to-batch consistency of solid oral dosage forms such as tablets, and drug development, i.e., to predict in vivo drug release profiles. There are three typical situations where dissolution testing plays a vital role: (i) formulation and optimization decisions: during product development, for products where dissolution performance is a critical quality attribute, both the product formulation and the manufacturing process are optimized based on achieving specific dissolution targets. (ii) Equivalence decisions: during generic product development, and also when implementing post-approval process or formulation changes, similarity of in vitro dissolution profiles between the reference product and its generic or modified version are one of the key requirements for regulatory approval decisions. (iii) Product compliance and release decisions: during routine manufacturing, dissolution outcomes are very often one of the criteria used to make product release decisions.
Dose dumping is a phenomenon of drug metabolism in which environmental factors can cause the premature and exaggerated release of a drug. This can greatly increase the concentration of a drug in the body and thereby produce adverse effects or even drug-induced toxicity.

Rubitecan is an oral topoisomerase inhibitor, developed by SuperGen.
Mucoadhesion describes the attractive forces between a biological material and mucus or mucous membrane. Mucous membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are generally hydrophilic as they contain many hydrogen macromolecules due to the large amount of water within its composition. However, mucin also contains glycoproteins that enable the formation of a gel-like substance. Understanding the hydrophilic bonding and adhesion mechanisms of mucus to biological material is of utmost importance in order to produce the most efficient applications. For example, in drug delivery systems, the mucus layer must be penetrated in order to effectively transport micro- or nanosized drug particles into the body. Bioadhesion is the mechanism by which two biological materials are held together by interfacial forces. The mucoadhesive properties of polymers can be evaluated via rheological synergism studies with freshly isolated mucus, tensile studies and mucosal residence time studies. Results obtained with these in vitro methods show a high correlation with results obtained in humans.
A powder is an assembly of dry particles dispersed in air. If two different powders are mixed perfectly, theoretically, three types of powder mixtures can be obtained: the random mixture, the ordered mixture or the interactive mixture.
Ciclosporin is a cyclic polypeptide that has been used widely as an orally-available immunosuppressant. It was originally used to prevent transplant rejection of solid organs but has also found use as an orally administered agent to treat psoriasis, rheumatoid arthritis, dry eye and other auto-immune related conditions. A variety of pre-clinical and clinical studies have been and are investigating its use to treat lung-related disorders via inhalation.

Lipid nanoparticles (LNPs) are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle.
Buccal administration is a topical route of administration by which drugs held or applied in the buccal area diffuse through the oral mucosa and enter directly into the bloodstream. Buccal administration may provide better bioavailability of some drugs and a more rapid onset of action compared to oral administration because the medication does not pass through the digestive system and thereby avoids first pass metabolism.

OSU-03012 (AR-12) is a celecoxib derivative with anticancer and anti-microbial activity. Unlike celecoxib, OSU-03012 does not inhibit COX, but inhibits several other important enzymes instead which may be useful in the treatment of some forms of cancer, When combined with PDE5 inhibitors such as sildenafil or tadalafil, OSU-03012 was found to show enhanced anti-tumour effects in cell culture.

Gabapentinoids, also known as α2δ ligands, are a class of drugs that are derivatives of the inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) which block α2δ subunit-containing voltage-dependent calcium channels (VDCCs). This site has been referred to as the gabapentin receptor, as it is the target of the drugs gabapentin and pregabalin.

Andreas Bernkop-Schnürch is an Austrian pharmaceutical technologist, scientist, pharmacist, entrepreneur, inventor and professor at the Institute of Pharmacy, University of Innsbruck. His research centers on the areas of pharmaceutical sciences, drug delivery, controlled release, bionanotechnology and polymer engineering. He is the inventor of various technologies such as thiolated polymers for that he coined the name thiomers in 2000 and phosphatase triggered charge converting nanoparticles for mucosal drug delivery. From 2016 to 2018 he served as a member of the Scientific Committee of the Innovative Medicines Initiative (IMI) of the European Union in Brussels giving advice on scientific priorities to be included in the Strategic Research Agenda for Horizon 2020. Since 2014 he is on the scientific advisory board of the Nicotine Science Center, Denmark. Andreas Bernkop-Schnürch is the founder of Mucobiomer Biotechnologische Forschungs- und Entwicklungs GmbH, Thiomatrix Forschungs- und Beratungs GmbH and Green River Polymers Forschungs und Entwicklungs GmbH. He is listed as a Highly Cited Researcher of the Institute of Scientific Information.

Ethosomes are phospholipid nanovesicles used for dermal and transdermal delivery of molecules. Ethosomes were developed by Touitou et al.,1997, as additional novel lipid carriers composed of ethanol, phospholipids, and water. They are reported to improve the skin delivery of various drugs. Ethanol is an efficient permeation enhancer that is believed to act by affecting the intercellular region of the stratum corneum. Ethosomes are soft malleable vesicles composed mainly of phospholipids, ethanol, and water. These soft vesicles represent novel vesicles carriers for enhanced delivery through the skin. The size of the ethosomes vesicles can be modulated from tens of nanometers to microns.
Professor Alastair J Sloan is an applied bioscientist and expert in the broad field of mineralised connective tissues, and since January 2020 current head of the Melbourne Dental School, University of Melbourne.
Protein nanotechnology is a burgeoning field of research that integrates the diverse physicochemical properties of proteins with nanoscale technology. This field assimilated into pharmaceutical research to give rise to a new classification of nanoparticles termed protein nanoparticles (PNPs). PNPs garnered significant interest due to their favorable pharmacokinetic properties such as high biocompatibility, biodegradability, and low toxicity Together, these characteristics have the potential to overcome the challenges encountered with synthetic NPs drug delivery strategies. These existing challenges including low bioavailability, a slow excretion rate, high toxicity, and a costly manufacturing process, will open the door to considerable therapeutic advancements within oncology, theranostics, and clinical translational research.
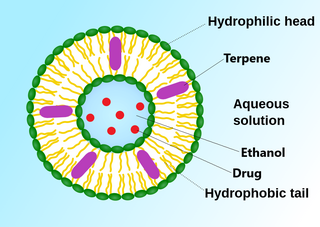
An invasome are a type of artificial vesicle nanocarrier that transport substances through the skin, the most superficial biological barrier. Vesicles are small particles surrounded by a lipid layer that can carry substances into and out of the cell. Artificial vesicles can be engineered to deliver drugs within the cell, with specific applications within transdermal drug delivery. However, the skin proves to be a barrier to effective penetration and delivery of drug therapies. Thus, invasomes are a new generation of vesicle with added structural components to assist with skin penetration.
References
- 1 2 Spernath A; Aserin A (December 2006). "Microemulsions as carriers for drugs and nutraceuticals". Adv Colloid Interface Sci. 128–130: 47–64. doi:10.1016/j.cis.2006.11.016. PMID 17229398.
- ↑ Jing Q; Shen Y; Ren F; Chen J; Jiang Z; Peng B; Leng Y; Dong J (November 2006). "HPLC determination of anethole trithione and its application to pharmacokinetics in rabbits". J Pharm Biomed Anal. 42 (5): 613–7. doi:10.1016/j.jpba.2006.05.013. PMID 16824723.
- ↑ Zhang P; Liu Y; Feng N; Xu J (May 2008). "Preparation and evaluation of self-microemulsifying drug delivery system of oridonin". Int J Pharm. 355 (1–2): 269–76. doi:10.1016/j.ijpharm.2007.12.026. PMID 18242895.
- ↑ Liu Y; Zhang P; Feng NP; Zhang X; Xu J (September 2008). "[Release kinetics of oridonin self-microemulsifying drug delivery system in vitro]". Zhongguo Zhong Yao Za Zhi (in Chinese). 33 (18): 2049–52. PMID 19160780.
- ↑ Liu Y; Zhang P; Feng N; Zhang X; Wu S; Zhao J (January 2009). "Optimization and in situ intestinal absorption of self-microemulsifying drug delivery system of oridonin". Int J Pharm. 365 (1–2): 136–42. doi:10.1016/j.ijpharm.2008.08.009. PMID 18782611.
- ↑ Cui J; Yu B; Zhao Y; Zhu W; Li H; Lou H; Zhai G (December 2008). "Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems". Int J Pharm. 371 (1–2): 148–55. doi:10.1016/j.ijpharm.2008.12.009. PMID 19124065.
- ↑ Chen Y; Li G; Wu X; Chen Z; Hang J; Qin B; Chen S; Wang R (January 2008). "Self-microemulsifying drug delivery system (SMEDDS) of vinpocetine: formulation development and in vivo assessment". Biol. Pharm. Bull. 31 (1): 118–25. doi: 10.1248/bpb.31.118 . PMID 18175953.
- ↑ Cui SX; Nie SF; Li L; Wang CG; Pan WS; Sun JP (November 2008). "Preparation and Evaluation of Self-Microemulsifying Drug Delivery System Containing Vinpocetine". Drug Dev Ind Pharm. 35 (5): 603–611. doi:10.1080/03639040802488089. PMID 19040178.
- ↑ Borhade VB; Nair HA; Hegde DD (October 2008). "Development and Characterization of Self-Microemulsifying Drug Delivery System of Tacrolimus for Intravenous Administration". Drug Dev Ind Pharm. 35 (5): 619–630. doi:10.1080/03639040802498856. PMID 18979309. S2CID 7127624.
- ↑ Borhade V; Nair H; Hegde D (2008). "Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus". AAPS PharmSciTech. 9 (1): 13–21. doi:10.1208/s12249-007-9014-8. PMC 2976874 . PMID 18446456.
- ↑ Borhade VB; Nair HA; Hegde DD; Barhate CR (November 2008). "Development and Validation of HPTLC Method for Estimation of Tacrolimus in Formulations". Drug Dev Ind Pharm. 35 (4): 440–448. doi:10.1080/03639040802430594. PMID 19040177. S2CID 97608266.
- ↑ Zhang BE; Lu WB; Chen WW (July 2008). "[Study on self-microemulsifying drug delivery system of Jiaotai Pill active components]". Zhong Yao Cai (in Chinese). 31 (7): 1068–71. PMID 18973026.
- ↑ Yao J; Lu Y; Zhou JP (2008). "Preparation of nobiletin in self-microemulsifying systems and its intestinal permeability in rats". J Pharm Pharm Sci. 11 (3): 22–9. doi: 10.18433/J3MS3M . PMID 18801304. Archived from the original on 2011-07-06. Retrieved 2009-03-13.
- ↑ Attama AA; Nkemnele MO (November 2005). "In vitro evaluation of drug release from self micro-emulsifying drug delivery systems using a biodegradable homolipid from Capra hircus". Int J Pharm. 304 (1–2): 4–10. doi:10.1016/j.ijpharm.2005.08.018. PMID 16198521.
- ↑ Zhou XT; Wang J; Wang Y; Sun JY; Nie SF; Pan WS (April 2008). "[Design and in vitro evaluation of self-microemulsifying drug delivery systems for piroxicam]". Yao Xue Xue Bao (in Chinese). 43 (4): 415–20. PMID 18664206.
- ↑ Mandawgade SD; Sharma S; Pathak S; Patravale VB (October 2008). "Development of SMEDDS using natural lipophile: application to beta-Artemether delivery". Int J Pharm. 362 (1–2): 179–83. doi:10.1016/j.ijpharm.2008.06.021. PMID 18652886.
- ↑ Holm R; Porter CJ; Edwards GA; Müllertz A; Kristensen HG; Charman WN (September 2003). "Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides". Eur J Pharm Sci. 20 (1): 91–7. doi:10.1016/S0928-0987(03)00174-X. PMID 13678797.
- 1 2 Ljusberg-Wahren H; Seier Nielsen F; Brogård M; Troedsson E; Müllertz A (July 2005). "Enzymatic characterization of lipid-based drug delivery systems". Int J Pharm. 298 (2): 328–32. doi:10.1016/j.ijpharm.2005.02.038. PMID 15979260.
- 1 2 Goddeeris C; Coacci J; Van den Mooter G (May 2007). "Correlation between digestion of the lipid phase of smedds and release of the anti-HIV drug UC 781 and the anti-mycotic drug enilconazole from smedds". Eur J Pharm Biopharm. 66 (2): 173–81. doi:10.1016/j.ejpb.2006.10.005. PMID 17158039.
- ↑ Goddeeris C; Van den Mooter G (September 2008). "Free flowing solid dispersions of the anti-HIV drug UC 781 with Poloxamer 407 and a maximum amount of TPGS 1000: investigating the relationship between physicochemical characteristics and dissolution behaviour". Eur J Pharm Sci. 35 (1–2): 104–13. doi:10.1016/j.ejps.2008.06.010. PMID 18644442.
- 1 2 3 Yi T; Wan J; Xu H; Yang X (October 2008). "A new solid self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs". Eur J Pharm Biopharm. 70 (2): 439–44. doi:10.1016/j.ejpb.2008.05.001. PMID 18603415.
- ↑ Yi T; Wan J; Xu H; Yang X (August 2008). "Controlled poorly soluble drug release from solid self-microemulsifying formulations with high viscosity hydroxypropylmethylcellulose". Eur J Pharm Sci. 34 (4–5): 274–80. doi: 10.1016/j.ejps.2008.04.010 . PMID 18541418.
- ↑ Singh AK; Chaurasiya A; Singh M; Upadhyay SC; Mukherjee R; Khar RK (2008). "Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization". AAPS PharmSciTech. 9 (2): 628–34. doi:10.1208/s12249-008-9080-6. PMC 2976939 . PMID 18473177.
- ↑ Lu JL; Wang JC; Zhao SX; Liu XY; Zhao H; Zhang X; Zhou SF; Zhang Q (August 2008). "Self-microemulsifying drug delivery system (SMEDDS) improves anticancer effect of oral 9-nitrocamptothecin on human cancer xenografts in nude mice". Eur J Pharm Biopharm. 69 (3): 899–907. doi:10.1016/j.ejpb.2008.02.023. PMID 18434109.
- ↑ Yang S; Gursoy RN; Lambert G; Benita S (February 2004). "Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors" (PDF). Pharm. Res. 21 (2): 261–70. doi:10.1023/B:PHAM.0000016238.44452.f1. PMID 15032307. S2CID 24590025.
- ↑ Kang BK; Chon SK; Kim SH; Jeong SY; Kim MS; Cho SH; Lee HB; Khang G (November 2004). "Controlled release of paclitaxel from microemulsion containing PLGA and evaluation of anti-tumor activity in vitro and in vivo". Int J Pharm. 286 (1–2): 147–56. doi:10.1016/j.ijpharm.2004.08.008. PMID 15501011.
- ↑ Grove M; Müllertz A; Nielsen JL; Pedersen GP (June 2006). "Bioavailability of seocalcitol II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides". Eur J Pharm Sci. 28 (3): 233–42. doi:10.1016/j.ejps.2006.02.005. PMID 16650738.
- ↑ Grove M; Müllertz A; Pedersen GP; Nielsen JL (May 2007). "Bioavailability of seocalcitol III. Administration of lipid-based formulations to minipigs in the fasted and fed state". Eur J Pharm Sci. 31 (1): 8–15. doi:10.1016/j.ejps.2007.01.007. PMID 17383165.
- ↑ Lee S; Lee J; Choi YW (April 2008). "Design and evaluation of prostaglandin E1 (PGE1) intraurethral liquid formulation employing self-microemulsifying drug delivery system (SMEDDS) for erectile dysfunction treatment". Biol. Pharm. Bull. 31 (4): 668–72. doi: 10.1248/bpb.31.668 . PMID 18379060.
- ↑ Fatouros DG; Nielsen FS; Douroumis D; Hadjileontiadis LJ; Mullertz A (August 2008). "In vitro-in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks". Eur J Pharm Biopharm. 69 (3): 887–98. doi:10.1016/j.ejpb.2008.01.022. PMID 18367386.
- ↑ Woo JS; Song YK; Hong JY; Lim SJ; Kim CK (February 2008). "Reduced food-effect and enhanced bioavailability of a self-microemulsifying formulation of itraconazole in healthy volunteers". Eur J Pharm Sci. 33 (2): 159–65. doi:10.1016/j.ejps.2007.11.001. PMID 18178070.
- ↑ Patel AR; Vavia PR (2007). "Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate". AAPS J. 9 (3): E344–52. doi:10.1208/aapsj0903041. PMC 2751486 . PMID 18170981.
- ↑ Patel D; Sawant KK (December 2007). "Oral bioavailability enhancement of acyclovir by self-microemulsifying drug delivery systems (SMEDDS)". Drug Dev Ind Pharm. 33 (12): 1318–26. doi:10.1080/03639040701385527. PMID 18097805. S2CID 3119405.
- ↑ Kang BK; Lee JS; Chon SK; Jeong SY; Yuk SH; Khang G; Lee HB; Cho SH (April 2004). "Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs". Int J Pharm. 274 (1–2): 65–73. doi:10.1016/j.ijpharm.2003.12.028. PMID 15072783.
- ↑ Meng J; Zheng L (September 2007). "Application of mixture experimental design to simvastatin apparent solubility predictions in the microemulsifion formed by self-microemulsifying". Drug Dev Ind Pharm. 33 (9): 927–31. doi:10.1080/03639040601003733. PMID 17891578. S2CID 37334831.
- 1 2 Cirri M; Mura P; Mora PC (August 2007). "Liquid spray formulations of xibornol by using self-microemulsifying drug delivery systems". Int J Pharm. 340 (1–2): 84–91. doi:10.1016/j.ijpharm.2007.03.021. PMID 17531411.
- ↑ Wu W; Wang Y; Que L (July 2006). "Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system". Eur J Pharm Biopharm. 63 (3): 288–94. doi:10.1016/j.ejpb.2005.12.005. PMID 16527467.
- ↑ Woo JS; Kim TS; Park JH; Chi SC (January 2007). "Formulation and biopharmaceutical evaluation of silymarin using SMEDDS". Arch. Pharm. Res. 30 (1): 82–9. doi:10.1007/BF02977782. PMID 17328246. S2CID 22169992.
- ↑ Wang DK; Shi ZH; Liu L; Wang XY; Zhang CX; Zhao P (2006). "Development of self-microemulsifying drug delivery systems for oral bioavailability enhancement of alpha-Asarone in beagle dogs". PDA J Pharm Sci Technol. 60 (6): 343–9. PMID 17260899.
- ↑ Cui S; Zhao C; Chen D; He Z (May 2005). "Self-microemulsifying drug delivery systems (SMEDDS) for improving in vitro dissolution and oral absorption of Pueraria lobata isoflavone". Drug Dev Ind Pharm. 31 (4–5): 349–56. doi:10.1081/DDC-54309. PMID 16093200. S2CID 9408964.
- ↑ Cui S; Zhao C; Tang X; Chen D; He Z (June 2005). "Study on the bioavailability of puerarin from Pueraria lobata isoflavone self-microemulsifying drug-delivery systems and tablets in rabbits by liquid chromatography-mass spectrometry". Biomed. Chromatogr. 19 (5): 375–8. doi:10.1002/bmc.460. PMID 15627278.
- 1 2 Yu AH; Zhai GX; Cui J; Liu H (August 2006). "[Preparation of puerarin solid self-microemulsion]". Zhong Yao Cai (in Chinese). 29 (8): 834–8. PMID 17076244.
- ↑ Cui SM; Zhao CS; He ZG (June 2007). "[Assessment of Pueraria lobata isoflavone with self-microemulsifying drug delivery systems in vitro and in vivo]". Zhong Yao Cai (in Chinese). 30 (6): 684–7. PMID 17918441.
- ↑ Shen HR; Li ZD; Zhong MK (November 2005). "[Preparation and evaluation of self-microemulsifying drug delivery systems containing atorvastatin]". Yao Xue Xue Bao (in Chinese). 40 (11): 982–7. PMID 16499080.
- ↑ Shen HR; Li ZD; Zhong MK (January 2006). "HPLC assay and pharmacokinetic study of atorvastatin in beagle dogs after oral administration of atorvastatin self-microemulsifying drug delivery system". Pharmazie. 61 (1): 18–20. PMID 16454200.
- ↑ Shen H; Zhong M (September 2006). "Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin". J. Pharm. Pharmacol. 58 (9): 1183–91. doi:10.1211/jpp.58.9.0004. PMID 16945176. S2CID 22542504.
- 1 2 Ito Y; Kusawake T; Prasad YV; Sugioka N; Shibata N; Takada K (July 2006). "Preparation and evaluation of oral solid heparin using emulsifier and adsorbent for in vitro and in vivo studies". Int J Pharm. 317 (2): 114–9. doi:10.1016/j.ijpharm.2006.02.056. PMID 16631328.
- ↑ Wei L; Sun P; Nie S; Pan W (September 2005). "Preparation and evaluation of SEDDS and SMEDDS containing carvedilol". Drug Dev Ind Pharm. 31 (8): 785–94. doi:10.1080/03639040500216428. PMID 16221613. S2CID 12794172.
- ↑ Heo MY; Piao ZZ; Kim TW; Cao QR; Kim A; Lee BJ (May 2005). "Effect of solubilizing and microemulsifying excipients in polyethylene glycol 6000 solid dispersion on enhanced dissolution and bioavailability of ketoconazole". Arch. Pharm. Res. 28 (5): 604–11. doi:10.1007/BF02977766. PMID 15974450. S2CID 11978203.
- ↑ Ito Y; Kusawake T; Ishida M; Tawa R; Shibata N; Takada K (June 2005). "Oral solid gentamicin preparation using emulsifier and adsorbent". J Control Release. 105 (1–2): 23–31. doi:10.1016/j.jconrel.2005.03.017. PMID 15908031.
- ↑ Sha X; Yan G; Wu Y; Li J; Fang X (April 2005). "Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells". Eur J Pharm Sci. 24 (5): 477–86. doi:10.1016/j.ejps.2005.01.001. PMID 15784337.
- ↑ Li P; Ghosh A; Wagner RF; Krill S; Joshi YM; Serajuddin AT (January 2005). "Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions". Int J Pharm. 288 (1): 27–34. doi:10.1016/j.ijpharm.2004.08.024. PMID 15607255.
- ↑ Subramanian N; Ray S; Ghosal SK; Bhadra R; Moulik SP (December 2004). "Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib". Biol. Pharm. Bull. 27 (12): 1993–9. doi: 10.1248/bpb.27.1993 . PMID 15577219.
- ↑ Porter CJ; Kaukonen AM; Boyd BJ; Edwards GA; Charman WN (August 2004). "Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation" (PDF). Pharm. Res. 21 (8): 1405–12. doi:10.1023/B:PHAM.0000036914.22132.cc. PMID 15359575. S2CID 8763220.
- ↑ Postolache P; Petrescu O; Dorneanu V; Zanini AC (2002). "Cyclosporine bioavailability of two physically different oral formulations". Eur Rev Med Pharmacol Sci. 6 (6): 127–31. PMID 12776806.
- ↑ Kim HJ; Yoon KA; Hahn M; Park ES; Chi SC (May 2000). "Preparation and in vitro evaluation of self-microemulsifying drug delivery systems containing idebenone". Drug Dev Ind Pharm. 26 (5): 523–9. doi:10.1081/DDC-100101263. PMID 10789064. S2CID 32771754.
- ↑ Gibaud, S. P.; Attivi, D. (2012). "Microemulsions for oral administration and their therapeutic applications" (PDF). Expert Opinion on Drug Delivery. 9 (8): 937–951. doi:10.1517/17425247.2012.694865. PMID 22663249. S2CID 28468973.
- ↑ Zupančič, O; Partenhauser, A; Lam, H Th; Rohrer, J; Bernkop-Schnürch, A (2016). "Development and in vitro characterisation of an oral self-emulsifying delivery system for daptomycin". European Journal of Pharmaceutical Sciences. 81: 129–136. doi:10.1016/j.ejps.2015.10.005. PMID 26485536.
- ↑ Leonaviciute, G; Bernkop-Schnürch, A (2015). "Self-emulsifying drug delivery systems in oral (poly)peptide drug delivery". Expert Opin Drug Deliv. 12 (11): 1703–1716. doi:10.1517/17425247.2015.1068287. PMID 26477549. S2CID 21890042.
- ↑ Friedl, H; Dünnhaupt, S; Hintzen, F; Waldner, C; Parikh, S; Pearson, JP; Wilcox, MD; Bernkop-Schnürch, A (2013). "Development and evaluation of a novel mucus diffusion test system approved by self-nanoemulsifying drug delivery systems". J Pharm Sci. 102 (12): 4406–4413. doi:10.1002/jps.23757. PMID 24258284.
- ↑ Sha, X; Yan, G; Wu, Y; Li, J; Fang, X (2005). "Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells". Eur J Pharm Sci. 24 (5): 477–486. doi:10.1016/j.ejps.2005.01.001. PMID 15784337.
- ↑ Hintzen, F; Perera, G; Hauptstein, S; Müller, C; Laffleur, F; Bernkop-Schnürch, A (2014). "In vivo evaluation of an oral self-microemulsifying drug delivery system (SMEDDS) for leuprorelin". Int J Pharm. 472 (1–2): 20–26. doi:10.1016/j.ijpharm.2014.05.047. PMID 24879935.





