Millets are a highly varied group of small-seeded grasses, widely grown around the world as cereal crops or grains for fodder and human food. Most millets belong to the tribe Paniceae.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Phyllostachys nigra, commonly known as black bamboo or purple bamboo, is a species of bamboo, native to Hunan Province of China, and is widely cultivated elsewhere.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

Daidzein is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones are produced in plants through the phenylpropanoid pathway of secondary metabolism and are used as signal carriers, and defense responses to pathogenic attacks. In humans, recent research has shown the viability of using daidzein in medicine for menopausal relief, osteoporosis, blood cholesterol, and lowering the risk of some hormone-related cancers, and heart disease. Despite the known health benefits, the use of both puerarin and daidzein is limited by their poor bioavailability and low water solubility.

Piceid is a stilbenoid glucoside and is a major resveratrol derivative in grape juices. It can be found in the bark of Picea sitchensis. It can also be isolated from Reynoutria japonica, the Japanese knotweed.

Olive leaf is the leaf of the olive tree. Although olive oil is well known for its flavor and possible health benefits, the leaf and its extracts remain under preliminary research with unknown effects on human health.

Flavonoids are synthesized by the phenylpropanoid metabolic pathway in which the amino acid phenylalanine is used to produce 4-coumaroyl-CoA. This can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalcones, which contain two phenyl rings. Conjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. The metabolic pathway continues through a series of enzymatic modifications to yield flavanones → dihydroflavonols → anthocyanins. Along this pathway, many products can be formed, including the flavonols, flavan-3-ols, proanthocyanidins (tannins) and a host of other various polyphenolics.

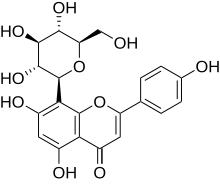
Orientin is a flavone, a chemical flavonoid-like compound. It is the 8-C glucoside of luteolin.

Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran and sugarcane.
The molecular formula C21H20O10 (molar mass: 432.38 g/mol, exact mass: 432.105647 u) may refer to:

Isovitexin is a flavone, namely the apigenin-6-C-glucoside. In this case, the prefix 'iso' does not imply an isoflavonoid, but the position of the glucoside on the flavone, in comparison to vitexin.

Cynaroside is a flavone, a flavonoid-like chemical compound. It is a 7-O-glucoside of luteolin.

Phyllostachys edulis, the mōsō bamboo, or tortoise-shell bamboo, or mao zhu, , is a temperate species of giant timber bamboo native to China and Taiwan and naturalised elsewhere, including Japan where it is widely distributed from south of Hokkaido to Kagoshima. The edulis part of the Latin name refers to its edible shoots. This bamboo can reach heights of up to 28 m (92 ft). This particular species of bamboo is the most common species used in the bamboo textile industry of China and other countries, for the production of rayon. Moso is less cold-hardy than many phyllostachys, surviving at a reduced height down to 5 degrees Fahrenheit (-15 °C).
Cunninghamella elegans is a species of fungus in the genus Cunninghamella found in soil.

Pteris ensiformis, the slender brake, silver lace fern, sword brake fern, or slender brake fern, is a plant species of the genus Pteris in the family Pteridaceae. It is found in Asia and the Pacific.

Liquiritin is the 4'-O-glucoside of the flavanone liquiritigenin. Liquiritin is one of flavone compounds derived from licorice.

Echinops echinatus, the Indian globe thistle, commonly known as Usnakantaka, is a species of globe thistle, found in India, Pakistan, and Sri Lanka. Indian globe thistle is an erect branched herb about 100 cm high. It has short, stout stems, branching from the base, covered with white cottony hair. Alternately arranged oblong, deeply pinnatifid leaves are 7–12 cm long. Flower heads occur in solitary white spherical balls, 3–5 cm across. Petals of the tiny white disc florets are 5 mm long. Flowers are surrounded by straight, strong, white bristles. Often misidentified with Silybum marianum (L.) Gaertner, it is colloquially known as Camel's thistle.

Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.

Phlomoides tuberosa, the sage-leaf mullein, is a perennial herbaceous flowering plant in the family Lamiaceae, native to China, Kazakhstan, Kyrgyzstan, Mongolia, Russia; SW Asia and Europe. Enlarged, tuberous roots give rise to erect stems to 150 cm bearing purple-red flowers.