 | |
 | |
| Names | |
|---|---|
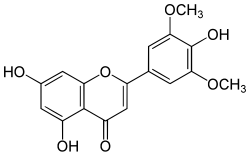
| IUPAC name 4′,5,7-Trihydroxy-3′,5′-dimethoxyflavone | |
| Systematic IUPAC name 5,7-Dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names Tricetin 3′,5′-dimethyl ether | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C17H14O7 | |
| Molar mass | 330.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran [1] and sugarcane. [2]
