The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1 to 2% of the pancreas volume and receive 10–15% of its blood flow. The pancreatic islets are arranged in density routes throughout the human pancreas, and are important in the metabolism of glucose.

Glucokinase is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role in the regulation of carbohydrate metabolism by acting as a glucose sensor, triggering shifts in metabolism or cell function in response to rising or falling levels of glucose, such as occur after a meal or when fasting. Mutations of the gene for this enzyme can cause unusual forms of diabetes or hypoglycemia.

Incretins are a group of metabolic hormones that stimulate a decrease in blood glucose levels. Incretins are released after eating and augment the secretion of insulin released from pancreatic beta cells of the islets of Langerhans by a blood glucose-dependent mechanism.

Gastric inhibitory polypeptide (GIP) or gastroinhibitory peptide, also known as the glucose-dependent insulinotropic peptide, is an inhibiting hormone of the secretin family of hormones. While it is weak inhibitor of gastric acid secretion, its main role is to stimulate insulin secretion.

Exenatide, sold under the brand name Byetta and Bydureon among others, is a medication used to treat diabetes mellitus type 2. It is used together with diet, exercise, and potentially other antidiabetic medication. It is a less preferred treatment option after metformin and sulfonylureas. It is given by injection under the skin within an hour before the first and last meal of the day. A once-weekly injection version is also avaliable.

The glucagon receptor is a 62 kDa protein that is activated by glucagon and is a member of the class B G-protein coupled family of receptors, coupled to G alpha i, Gs and to a lesser extent G alpha q. Stimulation of the receptor results in activation of adenylate cyclase and increased levels of intracellular cAMP. In humans, the glucagon receptor is encoded by the GCGR gene.
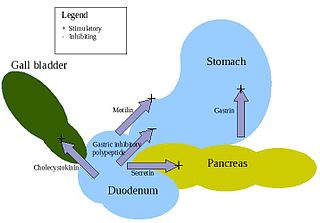
Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas.

The gastric inhibitory polypeptide receptor (GIP-R), also known as the glucose-dependent insulinotropic polypeptide receptor, is a protein that in humans is encoded by the GIPR gene. GIP-R is a member of the 7-transmembrane protein family, a class of G protein–coupled receptors. GIP-R is found on beta-cells in the pancreas.

Glucagon-like peptide-2 receptor (GLP-2R) is a protein that in human is encoded by the GLP2R gene located on chromosome 17.
Albiglutide is a glucagon-like peptide-1 agonist drug marketed by GlaxoSmithKline (GSK) for treatment of type 2 diabetes. As of 2017 it is unclear if it affects a person's risk of death. GSK has announced that it intends to withdraw the drug from the worldwide market by July 2018 for economic reasons.
Glucagon-like peptide-1 receptor agonists, also known as GLP-1 receptor agonists or incretin mimetics, are agonists of the GLP-1 receptor. This class of medications is used for the treatment of type 2 diabetes. One of their advantages over older insulin secretagogues, such as sulfonylureas or meglitinides, is that they have a lower risk of causing hypoglycemia.

Forkhead box protein O1 (FOXO1) also known as forkhead in rhabdomyosarcoma is a protein that in humans is encoded by the FOXO1 gene. FOXO1 is a transcription factor that plays important roles in regulation of gluconeogenesis and glycogenolysis by insulin signaling, and is also central to the decision for a preadipocyte to commit to adipogenesis. It is primarily regulated through phosphorylation on multiple residues; its transcriptional activity is dependent on its phosphorylation state.
The insulin transduction pathway is a biochemical pathway by which insulin increases the uptake of glucose into fat and muscle cells and reduces the synthesis of glucose in the liver and hence is involved in maintaining glucose homeostasis. This pathway is also influenced by fed versus fasting states, stress levels, and a variety of other hormones.
Dulaglutide is a glucagon-like peptide-1 receptor agonist consisting of GLP-1(7-37) covalently linked to an Fc fragment of human IgG4. It is used for the treatment of type 2 diabetes and can be used once weekly. GLP-1 is a hormone that is involved in the normalization of level of glucose in blood (glycemia). The FDA approved dulaglutide for use in the United States in September 2014. The drug is manufactured by Eli Lilly under the brand name Trulicity.

Semaglutide is a pharmaceutical drug developed by Danish company Novo Nordisk. Originally developed for the treatment of type 2 diabetes in 2012, it was found in 2017 that it can be used for the treatment of obesity.