Related Research Articles

The miR-8 microRNA precursor, is a short non-coding RNA gene involved in gene regulation. miR-8 in Drosophila melanogaster is expressed from the 3' arm of related precursor hairpins, along with miR-200, miR-236, miR-429 and human and mouse homolog miR-141. Members of this precursor family have now been predicted or experimentally confirmed in a wide range of species. The bounds of the precursors are predicted based on conservation and base pairing and are not generally known.

mir-133 is a type of non-coding RNA called a microRNA that was first experimentally characterised in mice. Homologues have since been discovered in several other species including invertebrates such as the fruitfly Drosophila melanogaster. Each species often encodes multiple microRNAs with identical or similar mature sequence. For example, in the human genome there are three known miR-133 genes: miR-133a-1, miR-133a-2 and miR-133b found on chromosomes 18, 20 and 6 respectively. The mature sequence is excised from the 3' arm of the hairpin. miR-133 is expressed in muscle tissue and appears to repress the expression of non-muscle genes.

The miR-199 microRNA precursor is a short non-coding RNA gene involved in gene regulation. miR-199 genes have now been predicted or experimentally confirmed in mouse, human and a further 21 other species. microRNAs are transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. The mature products are thought to have regulatory roles through complementarity to mRNA.

There are 89 known sequences today in the microRNA 19 (miR-19) family but it will change quickly. They are found in a large number of vertebrate species. The miR-19 microRNA precursor is a small non-coding RNA molecule that regulates gene expression. Within the human and mouse genome there are three copies of this microRNA that are processed from multiple predicted precursor hairpins:
The miR-34 microRNA precursor family are non-coding RNA molecules that, in mammals, give rise to three major mature miRNAs. The miR-34 family members were discovered computationally and later verified experimentally. The precursor miRNA stem-loop is processed in the cytoplasm of the cell, with the predominant miR-34 mature sequence excised from the 5' arm of the hairpin.
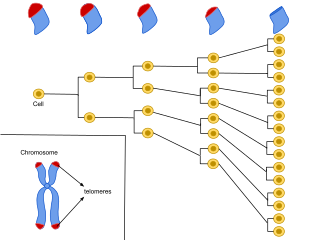
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.

microRNA 21 also known as hsa-mir-21 or miRNA21 is a mammalian microRNA that is encoded by the MIR21 gene.

MiR-155 is a microRNA that in humans is encoded by the MIR155 host gene or MIR155HG. MiR-155 plays a role in various physiological and pathological processes. Exogenous molecular control in vivo of miR-155 expression may inhibit malignant growth, viral infections, and enhance the progression of cardiovascular diseases.

In molecular biology mir-126 is a short non-coding RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several pre- and post-transcription mechanisms.

In molecular biology, miR-137 is a short non-coding RNA molecule that functions to regulate the expression levels of other genes by various mechanisms. miR-137 is located on human chromosome 1p22 and has been implicated to act as a tumor suppressor in several cancer types including colorectal cancer, squamous cell carcinoma and melanoma via cell cycle control.
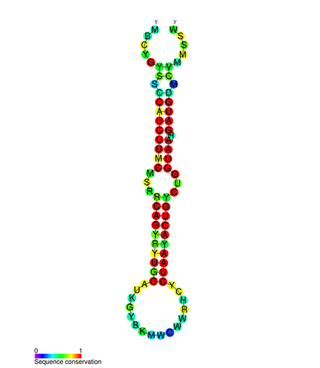
In molecular biology, the miR-200 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by binding and cleaving mRNAs or inhibiting translation. The miR-200 family contains miR-200a, miR-200b, miR-200c, miR-141, and miR-429. There is growing evidence to suggest that miR-200 microRNAs are involved in cancer metastasis.

In molecular biology miR-205 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. They are involved in numerous cellular processes, including development, proliferation, and apoptosis. Currently, it is believed that miRNAs elicit their effect by silencing the expression of target genes.

miR-138 is a family of microRNA precursors found in animals, including humans. MicroRNAs are typically transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. The excised region or, mature product, of the miR-138 precursor is the microRNA mir-138.
In molecular biology mir-339 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. miR-339-5p expression was associated with overall survival in breast cancer.
In molecular biology mir-345 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-367 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-503 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
In molecular biology mir-193 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.

MicroRNA 34a (miR-34a) is a MicroRNA that in humans is encoded by the MIR34A gene.
References
- ↑ Shin KK, Kim YJ, Hong CP, Yang JW, Bae YC, Jung JS (2012). "miR-598 induces replicative senescence in human adipose tissue-derived mesenchymal stem cells via silent information regulator 1". Mol Cell Biochem. 372 (1–2): 285. doi: 10.1007/s11010-012-1330-y . PMID 22581440.