
Aspergillus is a genus consisting of several hundred mold species found in various climates worldwide.

A conidium, sometimes termed an asexual chlamydospore or chlamydoconidium, is an asexual, non-motile spore of a fungus. The word conidium comes from the Ancient Greek word for dust, κόνις (kónis). They are also called mitospores due to the way they are generated through the cellular process of mitosis. They are produced exogenously. The two new haploid cells are genetically identical to the haploid parent, and can develop into new organisms if conditions are favorable, and serve in biological dispersal.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Aspergillus sydowii is a pathogenic fungus that causes several diseases in humans. It has been implicated in the death of sea fan corals in the Caribbean Sea.
Aspergillus penicillioides is a species of fungus in the genus Aspergillus, and is among the most xerophilic fungi.

Aspergillus versicolor is a slow-growing species of filamentous fungus commonly found in damp indoor environments and on food products. It has a characteristic musty odor associated with moldy homes and is a major producer of the hepatotoxic and carcinogenic mycotoxin sterigmatocystin. Like other Aspergillus species, A. versicolor is an eye, nose, and throat irritant.
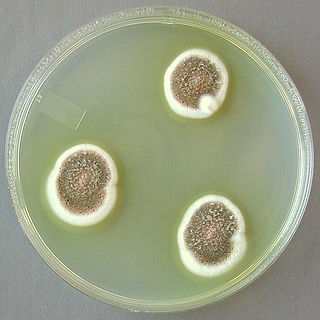
Aspergillus ustus is a microfungus and member of the division Ascomycota. It is commonly found in indoor environments and soil. Isolated cases of human infection resulting from A. ustus have been described; however the majority of these are nail infections.
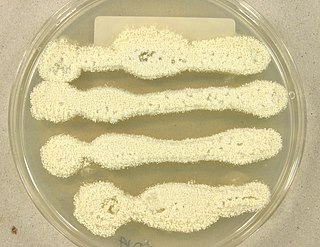
Aspergillus candidus is a white-spored species of fungus in the genus Aspergillus. Despite its lack of pigmentation, it is closely related to the most darkly-pigmented aspergilli in the Aspergillus niger group. It is a common soil fungus worldwide and is known as a contaminant of a wide array of materials from the indoor environment to foods and products. It is an uncommon agent of onychomycosis and aspergillosis. The species epithet candidus (L.) refers to the white pigmentation of colonies of this fungus. It is from the Candidi section. The fungi in the Candidi section are known for their white spores. It has been isolated from wheat flour, djambee, and wheat grain.
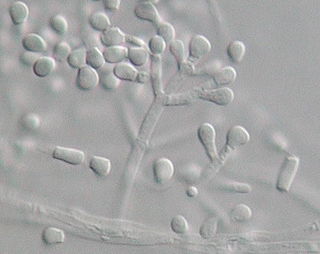
Geomyces pannorum is a yellow-brown filamentous fungus of the phylum Ascomycota commonly found in cold soil environments including the permafrost of the Northern hemisphere. A ubiquitous soil fungus, it is the most common species of the genus Geomyces; which also includes G. vinaceus and G. asperulatus. Geomyces pannorum has been identified as an agent of disfigurement of pigments used in the 15,000-year-old paintings on the walls of the Lascaux caves of France. Strains of Geomyces have been recovered from the Alaskan Fox Permafrost Tunnel and radiocarbon dated to between 14,000 and 30,000 years old.

Penicillium digitatum is a mesophilic fungus found in the soil of citrus-producing areas. It is a major source of post-harvest decay in fruits and is responsible for the widespread post-harvest disease in Citrus fruit known as green rot or green mould. In nature, this necrotrophic wound pathogen grows in filaments and reproduces asexually through the production of conidiophores and conidia. However, P. digitatum can also be cultivated in the laboratory setting. Alongside its pathogenic life cycle, P. digitatum is also involved in other human, animal and plant interactions and is currently being used in the production of immunologically based mycological detection assays for the food industry.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.

Aspergillus clavatus is a species of fungus in the genus Aspergillus with conidia dimensions 3–4.5 x 2.5–4.5 μm. It is found in soil and animal manure. The fungus was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.
Thermomyces lanuginosus is a species of thermophilic fungus that belongs to Thermomyces, a genus of hemicellulose degraders. It is classified as a deuteromycete and no sexual form has ever been observed. It is the dominant fungus of compost heaps, due to its ability to withstand high temperatures and use complex carbon sources for energy. As the temperature of compost heaps rises and the availability of simple carbon sources decreases, it is able to out compete pioneer microflora. It plays an important role in breaking down the hemicelluloses found in plant biomass due to the many hydrolytic enzymes that it produces, such as lipolase, amylase, xylanase, phytase, and chitinase. These enzymes have chemical, environmental, and industrial applications due to their hydrolytic properties. They are used in the food, petroleum, pulp and paper, and animal feed industries, among others. A few rare cases of endocarditis due to T. lanuginosus have been reported in humans.
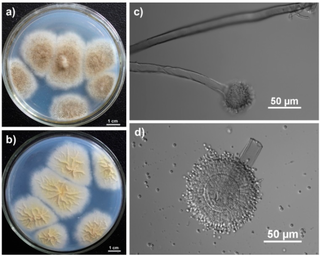
Aspergillus tubingensis is a darkly pigmented species of fungus in the genus Aspergillus section Nigri. It is often confused with Aspergillus niger due to their similar morphology and habitat. A. tubingensis is often involved in food spoilage of fruits and wheat, and industrial fermentation. This species is a rare agent of opportunistic infection.

Aspergillus parasiticus is a fungus belonging to the genus Aspergillus. This species is an unspecialized saprophytic mold, mostly found outdoors in areas of rich soil with decaying plant material as well as in dry grain storage facilities. Often confused with the closely related species, A. flavus, A. parasiticus has defined morphological and molecular differences. Aspergillus parasiticus is one of three fungi able to produce the mycotoxin, aflatoxin, one of the most carcinogenic naturally occurring substances. Environmental stress can upregulate aflatoxin production by the fungus, which can occur when the fungus is growing on plants that become damaged due to exposure to poor weather conditions, during drought, by insects, or by birds. In humans, exposure to A. parasiticus toxins can cause delayed development in children and produce serious liver diseases and/or hepatic carcinoma in adults. The fungus can also cause the infection known as aspergillosis in humans and other animals. A. parasiticus is of agricultural importance due to its ability to cause disease in corn, peanut, and cottonseed.

Penicillium spinulosum is a non-branched, fast-growing fungus with a swelling at the terminal of the stipe (vesiculate) in the genus Penicillium. P. spinulosum is able to grow and reproduce in environment with low temperature and low water availability, and is known to be acidotolerant. P. spinulosum is ubiquitously distributed, and can often be isolated from soil. Each individual strain of P. spinulosum differs from others in their colony morphology, including colony texture, amount of sporulation and roughness of conidia and conidiophores.

Alternaria brassicicola is a fungal necrotrophic plant pathogen that causes black spot disease on a wide range of hosts, particularly in the genus of Brassica, including a number of economically important crops such as cabbage, Chinese cabbage, cauliflower, oilseeds, broccoli and canola. Although mainly known as a significant plant pathogen, it also contributes to various respiratory allergic conditions such as asthma and rhinoconjunctivitis. Despite the presence of mating genes, no sexual reproductive stage has been reported for this fungus. In terms of geography, it is most likely to be found in tropical and sub-tropical regions, but also in places with high rain and humidity such as Poland. It has also been found in Taiwan and Israel. Its main mode of propagation is vegetative. The resulting conidia reside in the soil, air and water. These spores are extremely resilient and can overwinter on crop debris and overwintering herbaceous plants.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.
Aspergillus carneus is a fast-growing, filamentous fungus found on detritus and in fertile soil worldwide. It is characterized by its yellow, thick-walled hyphae and biseriate sterigmata. The fungus produces citrinin and 5 unique depsipeptides, Aspergillicins A-E.













