 | |
| Names | |
|---|---|
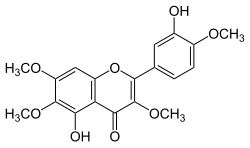
| IUPAC name 3′,5-Dihydroxy-3,4′,6,7-tetramethoxyflavone | |
| Systematic IUPAC name 5-Hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6,7-trimethoxy-4H-1-benzopyran-4-one | |
| Other names Vitexicarpin | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C19H18O8 | |
| Molar mass | 374.34 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Casticin is a methoxylated flavonol, meaning the core flavonoid structure has methyl groups attached. Found in Artemisia annua , the flavonoid has been shown to enhance the antimalarial activity of artemisinin though casticin itself has no direct antimalarial effects. [1] [2] It has been shown to have anti-mitotic activity. It is also found in Vitex agnus-castus . [3]