
Kaolinite is a clay mineral, part of the group of industrial minerals with the chemical composition Al2Si2O5(OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica (SiO
4) linked through oxygen atoms to one octahedral sheet of alumina (AlO
6) octahedra. Rocks that are rich in kaolinite are known as kaolin or china clay.

A mineral is, broadly speaking, a solid chemical compound that occurs naturally in pure form. Minerals are most commonly associated with rocks due to the presence of minerals within rocks. These rocks may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases. Compounds that occur only in living beings are usually excluded, but some minerals are often biogenic or are organic compounds in the sense of chemistry. Moreover, living beings often synthesize inorganic minerals that also occur in rocks.

In chemistry, a silicate is any member of a family of anions consisting of silicon and oxygen, usually with the general formula [SiO(4−2x)−
4−x]
n, where 0 ≤ x < 2. The family includes orthosilicate SiO4−
4, metasilicate SiO2−
3, and pyrosilicate Si
2O6−
7. The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate.

Gibbsite, Al(OH)3, is one of the mineral forms of aluminium hydroxide. It is often designated as γ-Al(OH)3 (but sometimes as α-Al(OH)3.). It is also sometimes called hydrargillite (or hydrargyllite).

Sekaninaite ((Fe+2,Mg)2Al4Si5O18) is a silicate mineral, the iron-rich analogue of cordierite.

Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of the Earth's crust.

Dickite is a phyllosilicate clay mineral named after the metallurgical chemist Allan Brugh Dick, who first described it. It is chemically composed of 20.90% aluminium, 21.76% silicon, 1.56% hydrogen and 55.78% oxygen. It has the same composition as kaolinite, nacrite, and halloysite, but with a different crystal structure (polymorph). Dickite sometimes contains impurities such as titanium, iron, magnesium, calcium, sodium and potassium.

Montmorillonite is a very soft phyllosilicate group of minerals that form when they precipitate from water solution as microscopic crystals, known as clay. It is named after Montmorillon in France. Montmorillonite, a member of the smectite group, is a 2:1 clay, meaning that it has two tetrahedral sheets of silica sandwiching a central octahedral sheet of alumina. The particles are plate-shaped with an average diameter around 1 μm and a thickness of 0.96 nm; magnification of about 25,000 times, using an electron microscope, is required to "see" individual clay particles. Members of this group include saponite.

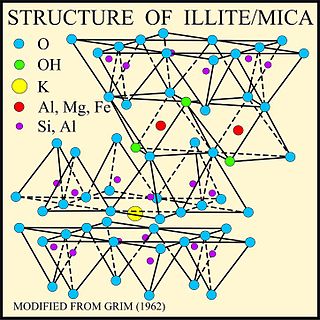
Illite is a group of closely related non-expanding clay minerals. Illite is a secondary mineral precipitate, and an example of a phyllosilicate, or layered alumino-silicate. Its structure is a 2:1 sandwich of silica tetrahedron (T) – alumina octahedron (O) – silica tetrahedron (T) layers. The space between this T-O-T sequence of layers is occupied by poorly hydrated potassium cations which are responsible for the absence of swelling. Structurally, illite is quite similar to muscovite with slightly more silicon, magnesium, iron, and water and slightly less tetrahedral aluminium and interlayer potassium. The chemical formula is given as (K,H
3O)(Al,Mg,Fe)
2(Si,Al)
4O
10[(OH)
2,(H
2O)], but there is considerable ion (isomorphic) substitution. It occurs as aggregates of small monoclinic grey to white crystals. Due to the small size, positive identification usually requires x-ray diffraction or SEM-EDS analysis. Illite occurs as an altered product of muscovite and feldspar in weathering and hydrothermal environments; it may be a component of sericite. It is common in sediments, soils, and argillaceous sedimentary rocks as well as in some low grade metamorphic rocks. The iron rich member of the illite group, glauconite, in sediments can be differentiated by x-ray analysis.

The tetrahedral-octahedral honeycomb, alternated cubic honeycomb is a quasiregular space-filling tessellation in Euclidean 3-space. It is composed of alternating regular octahedra and tetrahedra in a ratio of 1:2.
Expansive clay is a clay soil that is prone to large volume changes that are directly related to changes in water content. Soils with a high content of expansive minerals can form deep cracks in drier seasons or years; such soils are called vertisols. Soils with smectite clay minerals, including montmorillonite and bentonite, have the most dramatic shrink-swell capacity.
Metakaolin is the anhydrous calcined form of the clay mineral kaolinite. Minerals that are rich in kaolinite are known as china clay or kaolin, traditionally used in the manufacture of porcelain. The particle size of metakaolin is smaller than cement particles, but not as fine as silica fume.

Nontronite is the iron(III) rich member of the smectite group of clay minerals. Nontronites typically have a chemical composition consisting of more than ~30% Fe2O3 and less than ~12% Al2O3 (ignited basis). Nontronite has very few economic deposits like montmorillonite Like montmorillonite, nontronite can have variable amounts of adsorbed water associated with the interlayer surfaces and the exchange cations.
Brammallite is a sodium rich analogue of illite. First described in 1943 for an occurrence in Llandybie, Carmarthenshire, Wales, it was named for British geologist and mineralogist Alfred Brammall (1879–1954).
Zussmanite (K(Fe2+,Mg,Mn)13[AlSi17O42](OH)14) is a hydrated iron-rich silicate mineral. Zussmanite occurs as pale green crystals with perfect cleavage.

Iberulites are a particular type of microspherulites that develop in the atmosphere (troposphere), finally falling to the earth's surface. The name comes from the Iberian Peninsula where they were discovered.

Pimelite was discredited as a mineral species by the International Mineralogical Association (IMA) in 2006, in an article which suggests that “pimelite” specimens are probably willemseite, or kerolite. This was a mass discreditation, and not based on any re-examination of the type material. Nevertheless, a considerable number of papers have been written, verifying that pimelite is a nickel-dominant smectite. It is always possible to redefine a mineral wrongly discredited.
Illite crystallinity is a technique used to classify low-grade metamorphic activity in pelitic rocks. Determining the "illite crystallinity index" allows geologists to designate what metamorphic facies and metamorphic zone the rock was formed in and to infer what temperature the rock was formed. Several crystallinity indices have been proposed in recent years, but currently the Kübler index is being used due to its reproducibility and simplicity. The Kübler index is experimentally determined by measuring the full width at half maximum for the X-ray diffraction reflection peak along the (001) crystallographic axis of the rock sample. This value is an indirect measurement of the thickness of illite/muscovite packets which denote a change in metamorphic grade. The method can be used throughout the field of geology in areas such as the petroleum industry, plate tectonics.
Clay minerals are one of the most diverse minerals but all have a commonalty of crystal or grain sizes below 2 µm. Chemically clays are defined by crystal structure and chemical composition. Sometimes fine grain sediments are mistakenly described as clays, this is actually a description of the “clay-size fraction” rather than the mineralogy of the sediment. There are three crystallographic clay groups: platy clays (phyllosilicates), fibrous clay minerals, and amorphous clay. Phyllosilicates are the most abundant clays and are categorized based on the layering of a tetrahedral and an octahedral layer. For most clays, the octahedral layer is centered with Al3+, Fe3+, or Mg(OH)2, but sometimes Zn2+, Li+, and Cr3+ can substitute as well. Si4+ is normally the center of the tetrahedral layer but Al3+ will often partially substitute and create a charge imbalance. Two-layer clays are composed of a tetrahedral layer and an octahedral layer (T-O) while three-layer clays contain an octahedral layer sandwiched by two tetrahedral layers (T-O-T). When substitution of Al3+ for Si4+ creates a charge imbalance, an interlayer cation will fill in between tetrahedral layers to balance the charge of the clay.
Zigrasite is a phosphate mineral with the chemical formula of MgZr(PO4)2(H2O)4. Zigrasite was discovered and is only known to occur in the Dunton Quarry at Oxford County, Maine. Zigrasite was specifically found in the giant 1972 gem tourmaline-bearing pocket at the Dunton Quarry. Zigrasite is named after James Zigras who originally discovered and brought the mineral to attention.















