Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides which have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is used intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally as a treatment for severe Clostridium difficile colitis. When taken orally it is poorly absorbed.

Alamethicin is a channel-forming peptide antibiotic, produced by the fungus Trichoderma viride. It belongs to peptaibol peptides which contain the non-proteinogenic amino acid residue Aib. This residue strongly induces formation of alpha-helical structure. The peptide sequence is

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from Brevibacillus brevis soil bacteria. Gramicidins are linear peptides with 15 amino acids. This is in contrast to unrelated gramicidin S, which is a cyclic peptide.
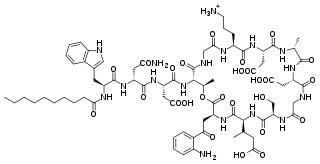
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.

The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of E. coli. In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Didemnins are cyclic depsipeptide compounds isolated from a tunicate of the genus Trididemnum that were collected in the Caribbean Sea. They were first isolated in 1978 at the University of Illinois.

The enzyme phenylalanine racemase is the enzyme that acts on amino acids and derivatives. It activates both the L & D stereo isomers of phenylalanine to form L-phenylalanyl adenylate and D-phenylalanyl adenylate, which are bound to the enzyme. These bound compounds are then transferred to the thiol group of the enzyme followed by conversion of its configuration, the D-isomer being the more favorable configuration of the two, with a 7 to 3 ratio between the two isomers. The racemisation reaction of phenylalanine is coupled with the highly favorable hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) and pyrophosphate (PP), thermodynamically allowing it to proceed. This reaction is then drawn forward by further hydrolyzing PP to inorganic phosphate (Pi), via Le Chatelier's principle.
Streptogramin B is a subgroup of the streptogramin antibiotics family. These natural products are cyclic hexa- or hepta depsipeptides produced by various members of the genus of bacteria Streptomyces. Many of the members of the streptogramins reported in the literature have the same structure and different names; for example, pristinamycin IA = vernamycin Bα = mikamycin B = osteogrycin B.
Gramicidin B is part of the collective Gramicidin D that is an antibiotic obtained from a soil microbe- Bacillus brevis. This antibiotic forms channels in the cell membrane through which cations inside the cell begin to leave, thus disrupting the ion potential and eventually killing the cell. Gramicidin B makes up 6% of Gramicidin D while Gramicidin A and C make up 80% and 14% respectively. Gramicidin D is a linear pentadecapeptide made up of 15 amino acids. The 11th amino acid in these chains leads to the three different types of gramicidins. Gramicindin A contains tryptophan in the 11th position while B and C have phenylalanine and tyrosine respectively.
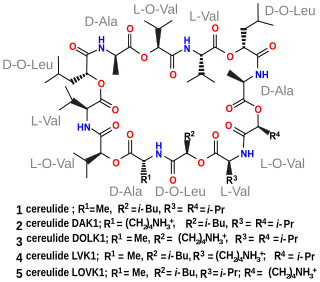
Cereulide is a toxin produced by some strains of Bacillus cereus, Bacillus megaterium and related species. It is a potent cytotoxin that destroys mitochondria. It also causes nausea and vomiting.
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
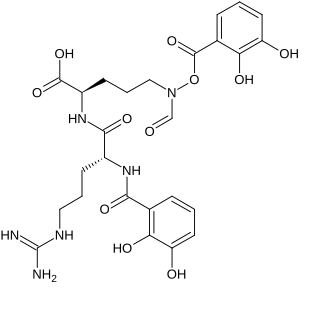
Mirubactin is a siderophore produced by the bacterium Actinosynnema mirum.A.mirum was first isolated from the Raritan River in New Jersey in 1976, and its full genome sequence was published in 2009. In 2012, mirubactin was isolated and characterized, and the biosynthesis was connected with the gene cluster Amir_2714-Amir_2728, since renamed mrbA-mrbO.
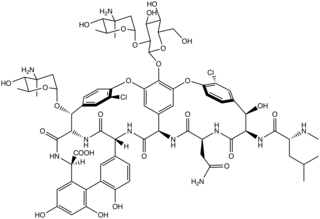
Chloroeremomycin is a member of the glycopeptide family of antibiotics, such as vancomycin. The molecule is a non-ribosomal polypeptide that has been glycosylated. It is composed of seven amino acids and three saccharide units. Although chloroeremomycin has never been in clinical phases, oritavancin, a semi-synthetic derivative of chloroeremomycin, has been investigated.
Andrimid is an antibiotic natural product that is produced by the marine bacterium Vibrio coralliilyticus. Andrimid is an inhibitor of fatty acid biosynthesis by blocking the carboxyl transfer reaction of acetyl-CoA carboxylase (ACC).

Halovir refers to a multi-analogue compound belonging to a group of oligopeptides designated as lipopeptaibols which have membrane-modifying capacity and are fungal in origin. These peptides display interesting microheterogeneity; slight variation in encoding amino acids gives rise to a mixture of closely related analogues and have been shown to have antibacterial/antiviral properties.
















