Related Research Articles

An alloy is a mixture of chemical elements of which in most cases at least one is a metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Most alloys are metallic and show good electrical conductivity, ductility, opacity, and luster, and may have properties that differ from those of the pure elements such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties such as corrosion resistance or mechanical strength.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Martensite is a very hard form of steel crystalline structure. It is named after German metallurgist Adolf Martens. By analogy the term can also refer to any crystal structure that is formed by diffusionless transformation.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.

In materials science, quenching is the rapid cooling of a workpiece in water, gas, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness.

Carburizing, or carburising, is a heat treatment process in which iron or steel absorbs carbon while the metal is heated in the presence of a carbon-bearing material, such as charcoal or carbon monoxide. The intent is to make the metal harder and more wear resistant. Depending on the amount of time and temperature, the affected area can vary in carbon content. Longer carburizing times and higher temperatures typically increase the depth of carbon diffusion. When the iron or steel is cooled rapidly by quenching, the higher carbon content on the outer surface becomes hard due to the transformation from austenite to martensite, while the core remains soft and tough as a ferritic and/or pearlite microstructure.

Maraging steels are steels that possess superior strength and toughness without losing ductility. Aging refers to the extended heat-treatment process. These steels are a special class of very-low-carbon ultra-high-strength steels that derive their strength from precipitation of intermetallic compounds rather than from carbon. The principal alloying metal is 15 to 25 wt% nickel. Secondary alloying metals, which include cobalt, molybdenum and titanium, are added to produce intermetallic precipitates.

Inconel is a nickel-chromium-based superalloy often utilized in extreme environments where components are subjected to high temperature, pressure or mechanical loads. Inconel alloys are oxidation- and corrosion-resistant. When heated, Inconel forms a thick, stable, passivating oxide layer protecting the surface from further attack. Inconel retains strength over a wide temperature range, attractive for high-temperature applications where aluminum and steel would succumb to creep as a result of thermally-induced crystal vacancies. Inconel's high-temperature strength is developed by solid solution strengthening or precipitation hardening, depending on the alloy.

Work hardening, also known as strain hardening, is the process by which a material's load-bearing capacity (strength) increases during plastic (permanent) deformation. This characteristic is what sets ductile materials apart from brittle materials. Work hardening may be desirable, undesirable, or inconsequential, depending on the application.
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels, stainless steels, and duplex stainless steel. In superalloys, it is known to cause yield strength anomaly providing excellent high-temperature strength.

Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered at much higher temperatures.
Cryogenic hardening is a cryogenic treatment process where the material is cooled to approximately −185 °C (−301 °F), typically using liquid nitrogen. It can have a profound effect on the mechanical properties of certain steels, provided their composition and prior heat treatment are such that they retain some austenite at room temperature. It is designed to increase the amount of martensite in the steel's crystal structure, increasing its strength and hardness, sometimes at the cost of toughness. Presently this treatment is being used on tool steels, high-carbon, high-chromium steels and in some cases to cemented carbide to obtain excellent wear resistance. Recent research shows that there is precipitation of fine carbides in the matrix during this treatment which imparts very high wear resistance to the steels.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.
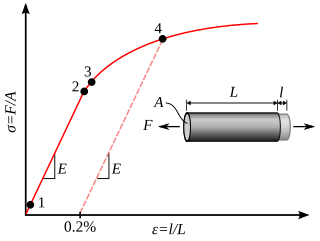
In materials science and engineering, the yield point is the point on a stress–strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible and is known as plastic deformation.
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling.
In materials science, hardness is a measure of the resistance to localized plastic deformation, such as an indentation or a scratch (linear), induced mechanically either by pressing or abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elastic stiffness, plasticity, strain, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are ceramics, concrete, certain metals, and superhard materials, which can be contrasted with soft matter.
In metallurgy, solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element to the crystalline lattice of another element, forming a solid solution. The local nonuniformity in the lattice due to the alloying element makes plastic deformation more difficult by impeding dislocation motion through stress fields. In contrast, alloying beyond the solubility limit can form a second phase, leading to strengthening via other mechanisms.
Methods have been devised to modify the yield strength, ductility, and toughness of both crystalline and amorphous materials. These strengthening mechanisms give engineers the ability to tailor the mechanical properties of materials to suit a variety of different applications. For example, the favorable properties of steel result from interstitial incorporation of carbon into the iron lattice. Brass, a binary alloy of copper and zinc, has superior mechanical properties compared to its constituent metals due to solution strengthening. Work hardening has also been used for centuries by blacksmiths to introduce dislocations into materials, increasing their yield strengths.

In materials science, grain-boundary strengthening is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. By changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.
Iron aluminides are intermetallic compounds of iron and aluminium - they typically contain ~18% Al or more.
References
- ↑ Hansen, Niels (2 June 2004). "Hall–Petch relation and boundary strengthening" (PDF). Center for Fundamental Research: Metal Structures in Four Dimensions, Materials Research Department, Risø National Laboratory. Retrieved 1 July 2024.
- ↑ "What is Work Hardening Steel? - Titus Steel". Titus Steel. 19 October 2023. Retrieved 1 July 2024.
