Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant, or as a wakefulness-promoting agent in higher doses.

Loratadine, sold under the brand name Claritin among others, is a medication used to treat allergies. This includes allergic rhinitis and hives. It is also available in combination with pseudoephedrine, a decongestant, known as loratadine/pseudoephedrine. It is taken by mouth.

Nifedipine, sold under the brand name Adalat among others, is a medication used to manage angina, high blood pressure, Raynaud's phenomenon, and premature labor. It is one of the treatments of choice for Prinzmetal angina. It may be used to treat severe high blood pressure in pregnancy. Its use in preterm labor may allow more time for steroids to improve the baby's lung function and provide time for transfer of the mother to a well qualified medical facility before delivery. Nifedipine is taken by mouth and comes in fast and slow release formulations.

A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. Often, this promotes healing to an injured area of the body. An advantage of a transdermal drug delivery route over other types of medication delivery such as oral, topical, intravenous, intramuscular, etc. is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier; as a result, only medications whose molecules are small enough to penetrate the skin can be delivered by this method. A wide variety of pharmaceuticals are now available in transdermal patch form.
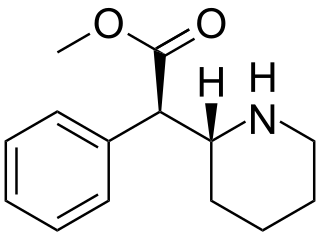
Dexmethylphenidate, sold under the brand name Focalin among others, is a medication used to treat attention deficit hyperactivity disorder (ADHD) in those over the age of 5 years. If no benefit is seen after 4 weeks it is reasonable to discontinue its use. It is taken by mouth. The immediate release formulation lasts up to 5 hours well the extended release formulation lasts up to 12 hours.

Baclofen, sold under the brand name Lioresal among others, is a medication used to treat muscle spasticity such as from a spinal cord injury or multiple sclerosis. It may also be used for hiccups and muscle spasms near the end of life. It is taken by mouth or by delivery into the spinal canal.
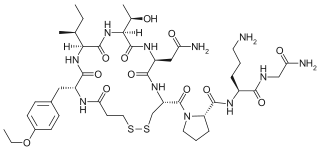
Atosiban is an inhibitor of the hormones oxytocin and vasopressin. It is used as an intravenous medication as a labour repressant (tocolytic) to halt premature labor. It was developed by Ferring Pharmaceuticals in Sweden and first reported in the literature in 1985. Originally marketed by Ferring Pharmaceuticals, it is licensed in proprietary and generic forms for the delay of imminent preterm birth in pregnant adult women. In India it is marketed under the brand name Tosiban by Zuventus healthcare ltd.

Phenylephrine is a medication primarily used as a decongestant, to dilate the pupil, to increase blood pressure, and to relieve hemorrhoids. While marketed as a decongestant, taken by mouth at recommended doses it is of unclear benefit for hay fever. It can be taken by mouth, given by injection into a vein or muscle, or applied to the skin.
Daytrana is a transdermal patch developed and marketed by Noven Pharmaceuticals, Inc. that was approved in April 2006. In the literature, Daytrana is most commonly referred to as methylphenidate transdermal system (MTS).

Drug delivery refers to approaches, formulations, technologies, and systems for transporting a pharmaceutical compound in the body as needed to safely achieve its desired therapeutic effect. It may involve scientific site-targeting within the body, or it might involve facilitating systemic pharmacokinetics; in any case, it is typically concerned with both quantity and duration of drug presence. Drug delivery is often approached via a drug's chemical formulation, but it may also involve medical devices or drug-device combination products. Drug delivery is a concept heavily integrated with dosage form and route of administration, the latter sometimes even being considered part of the definition.
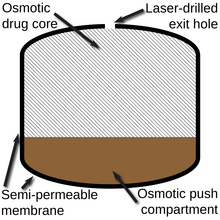
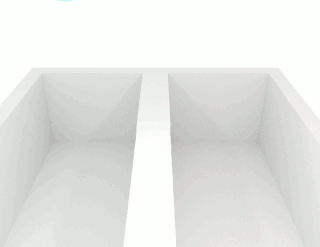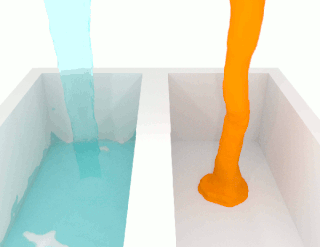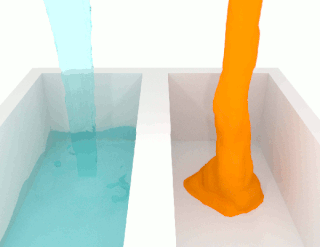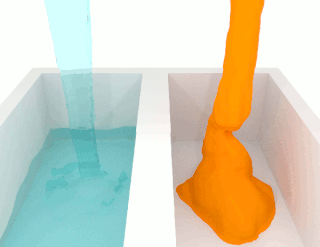
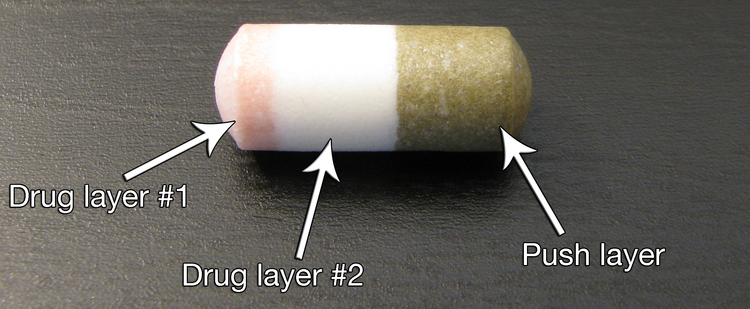
Modified-release dosage is a mechanism that delivers a drug with a delay after its administration or for a prolonged period of time or to a specific target in the body.
Attention deficit hyperactivity disorder management options are evidence-based practices with established treatment efficacy for ADHD. The American Academy of Pediatrics recommends different treatment paradigms depending on the age of the person being treated. For those aged 4–5, the Academy recommends evidence-based parent- and/or teacher-administered behavior therapy, with the addition of methylphenidate only if there is continuing moderate-to-severe functional disturbances. For those aged 6–11, the use of medication in combination with behavior therapy is recommended, with the evidence for stimulant medications being stronger than that for other classes. For those aged 12–18, medication should be prescribed with the consent of the treated adolescent, preferably in combination with behavioral therapy. The evidence for the utility of behavioral interventions in this aged group was rated only "C" quality, however.
Thiolated polymers or designated thiomers are experimental polymers used in biotechnology product development with the intent to enhance absorption of drugs. Thiomers have thiol bearing side chains. Thiomer compounds with low molecular mass are covalently bound to a polymeric backbone consisting of biodegradable polymers, such as chitosan, hyaluronic acid, gelatin, polyacrylates, cyclodextrins, or silicones.
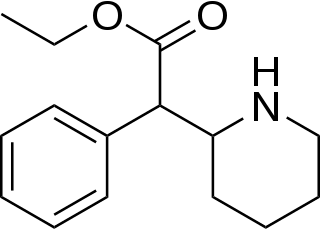
Ethylphenidate (EPH) is a psychostimulant and a close analog of methylphenidate.

Itopride (INN) (brand name Ganaton) is a prokinetic benzamide derivative unlike domperidone. These drugs inhibit dopamine and acetylcholine esterase enzyme and have a gastrokinetic effect. Itopride is indicated for the treatment of functional dyspepsia and other gastrointestinal conditions. It is a combined D2 receptor antagonist and acetylcholinesterase inhibitor.
Bioadhesion is the mechanism by which two biological materials are held together by interfacial forces. When relating this mechanism to the pharmaceutical sciences, mucoadhesion describes the attractive forces between a biological material and mucus or mucous membrane. Mucus membranes adhere to epithelial surfaces such as the gastrointestinal tract (GI-tract), the vagina, the lung, the eye, etc. They are generally hydrophilic as they contain many hydrogen macromolecules due to the large amount of water within its composition. However, mucin also contains glycoproteins that enable the formation of a gel-like substance. Understanding the hydrophilic bonding and adhesion mechanisms of mucus to biological material is of utmost importance in order to produce the most efficient applications. For example, in drug delivery systems, the mucus layer must be penetrated in order to effectively transport micro- or nanosized drug particles into the body.
Insufflation is the act of blowing something into a body cavity. Insufflation has many medical uses, most notably as a route of administration for various drugs.

4-Fluoromethylphenidate is a stimulant drug that acts as a higher potency dopamine reuptake inhibitor than the closely related methylphenidate.