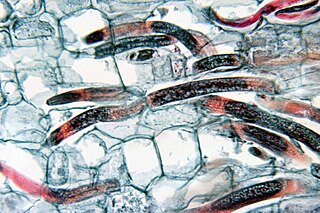
Radopholus similis is a species of nematode known commonly as the burrowing nematode. It is a parasite of plants, and it is a pest of many agricultural crops. It is an especially important pest of bananas, and it can be found on coconut, avocado, coffee, sugarcane, other grasses, and ornamentals. It is a migratory endoparasite of roots, causing lesions that form cankers. Infected plants experience malnutrition.

Meloidogyne incognita, also known as the southern root-nematode or cotton root-knot nematode is a plant-parasitic roundworm in the family Heteroderidae. This nematode is one of the four most common species worldwide and has numerous hosts. It typically incites large, usually irregular galls on roots as a result of parasitism.

Potato root nematodes or potato cyst nematodes (PCN) are 1-mm long roundworms belonging to the genus Globodera, which comprises around 12 species. They live on the roots of plants of the family Solanaceae, such as potatoes and tomatoes. PCN cause growth retardation and, at very high population densities, damage to the roots and early senescence of plants. The nematode is not indigenous to Europe but originates from the Andes. Fields are free from PCN until an introduction occurs, after which the typical patches, or hotspots, occur on the farmland. These patches can become full field infestations when unchecked. Yield reductions can average up to 60% at high population densities.
Belonolaimus longicaudatus is a common parasite of grasses and other plant crops and products. It is the most destructive nematode pest of turf grass, and it also attacks a wide range of fruit, vegetable, and fiber crops such as citrus, cotton, ornamentals, and forage. The sting nematode is a migratory ectoparasite of roots. It is well established in many golf courses and presents a problem in turf management. The sting nematode is only present in very sandy soils. It cannot reproduce in heavier or clay soils.

Meloidogyne arenaria is a species of plant pathogenic nematodes. This nematode is also known as the peanut root knot nematode. The word "Meloidogyne" is derived from two Greek words that mean "apple-shaped" and "female". The peanut root knot nematode, M. arenaria is one of the "major" Meloidogyne species because of its worldwide economic importance. M. arenaria is a predominant nematode species in the United States attacking peanut in Alabama, Florida, Georgia, and Texas. The most damaging nematode species for peanut in the USA is M. arenaria race 1 and losses can exceed 50% in severely infested fields. Among the several Meloidogyne species that have been characterized, M. arenaria is the most variable both morphologically and cytologically. In 1949, two races of this nematode had been identified, race 1 which reproduces on peanut and race 2 which cannot do so. However, in a recent study, three races were described. López-Pérez et al (2011) had also studied populations of M. arenaria race 2, which reproduces on tomato plants carrying the Mi gene and race 3, which reproduces on both resistant pepper and tomato.

Meloidogyne javanica is a species of plant-pathogenic nematodes. It is one of the tropical root-knot nematodes and a major agricultural pest in many countries. It has many hosts. Meloidogyne javanica reproduces by obligatory mitotic parthenogenesis (apomixis).
Pratylenchus brachyurus is a plant parasitic nematode.

Pratylenchus penetrans is a species of nematode in the genus Pratylenchus, the lesion nematodes. It occurs in temperate regions worldwide, regions between the subtropics and the polar circles. It is an animal that inhabits the roots of a wide variety of plants and results in necrotic lesions on the roots. Symptoms of P. penetrans make it hard to distinguish from other plant pathogens; only an assay of soil can conclusively diagnose a nematode problem in the field. P. penetrans is physically very similar to other nematode species, but is characterized by its highly distinctive mouthpiece. P. penetrans uses its highly modified mouth organs to rupture the outer surface of subterranean plant root structures. It will then enter into the root interior and feed on the plant tissue inside. P. penetrans is considered to be a crop parasite and farmers will often treat their soil with various pesticides in an attempt to eliminate the damage caused by an infestation. In doing this, farmers will also eliminate many of the beneficial soil fauna, which will lead to an overall degradation of soil quality in the future. Alternative, more environmentally sustainable methods to control P. penetrans populations may be possible in certain regions.

Pratylenchus zeae is a plant-pathogenic nematode found on potatoes, maize, cereal, tobacco, coffee, blackberry, and found most often on sugarcane.
Hirschmanniella oryzae, i.e. rice root nematode (RRN), is among the major pests of rice and is the most common plant-parasitic nematode found on irrigated rice. Recent modifications in cultivation practices have led to a substantial increase in rice production, which has been accompanied by heightened levels of RRN. The proportional increases in RRN with rice production can be explained by the nematode's impeccable adaptation towards constantly flooded conditions in which irrigated rice is often being grown.

Paratylenchus hamatus, the fig pin nematode, is a species of migratory plant endoparasites, that causes lesions on plant roots resulting in symptoms of chlorosis, wilting and ultimately yield losses. They move and feed on different parts of host tissue throughout their life cycle in order to find enough susceptible host tissue to survive and reproduce. A wide range of host plant species are susceptible to the fig pin nematode, including many valuable fruit and vegetable crops such as figs, carrots and celery. They are also commonly found associated with woody perennials in California. P. hamatus inhabits soils in both Europe and North America, and was originally isolated from fig in central California in 1950.
Xiphinema americanum, the American dagger nematode, is a species of plant pathogenic nematodes. It is one of many species that belongs to the genus Xiphinema. It was first described by N. A. Cobb in 1913, who found it on both sides of the United States on the roots of grass, corn, and citrus trees. Not only is Xiphinema americanum known to vector plant viruses, but also X. americanum has been referred to as "the most destructive plant parasitic nematode in America", and one of the four major nematode pests in the Southeastern United States.
Mesocriconema xenoplax is a species of plant parasitic nematodes. Nematodes of this particular species are collectively called ring nematodes.
Heterodera sacchari, the sugarcane cyst nematode, mitotic parthenogenic sedentary endoparasitic nematode. This plant-parasitic nematode infects the roots of sugarcane, and the female nematode eventually becomes a thick-walled cyst filled with eggs. Aboveground symptoms are species specific and are similar to those caused by other Heterodera species. Symptoms include: stunted and chlorotic plants, and reduced root growth. Seedlings may be killed in heavily infested soils.
Belonolaimus is a genus of nematodes. They are known commonly as sting nematodes. They are ectoparasites that feed on plant roots, sometimes becoming agricultural pests. They are found in the United States, Mexico, and Puerto Rico.
Hoplolaimus is a genus of nematodes known commonly as lance nematodes. They are parasites of plants, and three species are pests of agricultural crops.

Pratylenchus is a genus of nematodes known commonly as lesion nematodes. They are parasitic on plants and are responsible for root lesion disease on many taxa of host plants in temperate regions around the world. Lesion nematodes are migratory endoparasites that feed and reproduce in the root and move around, unlike the cyst or root-knot nematodes, which may stay in one place. They usually only feed on the cortex of the root. Species are distinguished primarily by the morphology of the stylets.
Helicotylenchus is a genus of nematodes in the family Hoplolaimidae. They are known generally as spiral nematodes. They are found worldwide because they can live and survive in a wide range of habitats. They are among the most common parasitic nematodes of plants; found in corn, bananas, grass, soybeans.
Pratylenchus alleni is a migratory endoparasitic nematode, living inside of plant roots and feeding on parenchyma cells in the root cortex. P. alleni is an obligate biotroph, meaning it must have a living host in order to survive. Due to their incredibly broad host range, Pratylenchus species fall third in total economic impact, finishing just behind cyst nematodes and root knot nematodes (Meloidogyne). In Canada, it was isolated for the first time in 2011 in a soybean field.
Heterodera zeae, the corn cyst nematode (CCN), is a plant parasitic nematode that feeds on Zea mays (maize/corn). The CCN has a limited economic impact worldwide due to its high soil temperature requirements.









