
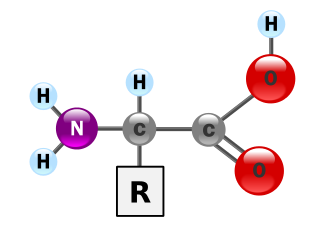
Lysine is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group, an α-carboxylic acid group, and a side chain (CH2)4NH2, and so it is classified as a basic, charged, aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

Amino acid biosynthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids.
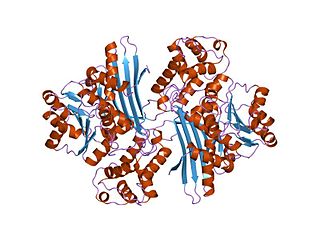
In molecular biology, the protein domain Saccharopine dehydrogenase (SDH), also named Saccharopine reductase, is an enzyme involved in the metabolism of the amino acid lysine, via an intermediate substance called saccharopine. The Saccharopine dehydrogenase enzyme can be classified under EC 1.5.1.7, EC 1.5.1.8, EC 1.5.1.9, and EC 1.5.1.10. It has an important function in lysine metabolism and catalyses a reaction in the alpha-Aminoadipic acid pathway. This pathway is unique to fungal organisms therefore, this molecule could be useful in the search for new antibiotics. This protein family also includes saccharopine dehydrogenase and homospermidine synthase. It is found in prokaryotes, eukaryotes and archaea.

In enzymology, a succinate-semialdehyde dehydrogenase (SSADH) (EC 1.2.1.24) is an enzyme that catalyzes the chemical reaction

In enzymology, an aminomuconate-semialdehyde dehydrogenase (EC 1.2.1.32) is an enzyme that catalyzes the chemical reaction

In enzymology, a L-aminoadipate-semialdehyde dehydrogenase (EC 1.2.1.31) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NAD+, L-glutamate-forming) (EC 1.5.1.9) is an enzyme that catalyzes the chemical reaction

In enzymology, a saccharopine dehydrogenase (NADP+, L-glutamate-forming) (EC 1.5.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NADP+, L-lysine-forming) (EC 1.5.1.8) is an enzyme that catalyzes the chemical reaction

The enzyme aminocarboxymuconate-semialdehyde decarboxylase (EC 4.1.1.45) catalyzes the chemical reaction

Succinate-semialdehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH5A1 gene.

Alpha-aminoadipic semialdehyde synthase is an enzyme encoded by the AASS gene in humans and is involved in their major lysine degradation pathway. It is similar to the separate enzymes coded for by the LYS1 and LYS9 genes in yeast, and related to, although not similar in structure, the bifunctional enzyme found in plants. In humans, mutations in the AASS gene, and the corresponding alpha-aminoadipic semialdehyde synthase enzyme are associated with familial hyperlysinemia. This rare disease is inherited in an autosomal recessive pattern and patients often have no clinical symptoms.

Methylmalonate-semialdehyde dehydrogenase [acylating], mitochondrial (MMSDH) is an enzyme that in humans is encoded by the ALDH6A1 gene.

L-aminoadipate-semialdehyde dehydrogenase-phosphopantetheinyl transferase is an enzyme that in humans is encoded by the AASDHPPT gene.
Inborn errors of amino acid metabolism are metabolic disorders which impair the synthesis and degradation of amino acids.

Aldehyde dehydrogenase 7 family, member A1, also known as ALDH7A1 or antiquitin, is an enzyme that in humans is encoded by the ALDH7A1 gene. The protein encoded by this gene is a member of subfamily 7 in the aldehyde dehydrogenase gene family. These enzymes are thought to play a major role in the detoxification of aldehydes generated by alcohol metabolism and lipid peroxidation. This particular member has homology to a previously described protein from the green garden pea, the 26g pea turgor protein. It is also involved in lysine catabolism that is known to occur in the mitochondrial matrix. Recent reports show that this protein is found both in the cytosol and the mitochondria, and the two forms likely arise from the use of alternative translation initiation sites. An additional variant encoding a different isoform has also been found for this gene. Mutations in this gene are associated with pyridoxine-dependent epilepsy. Several related pseudogenes have also been identified.

The α-aminoadipate pathway is a biochemical pathway for the synthesis of the amino acid L-lysine. In the eukaryotes, this pathway is unique to several species of yeast, higher fungi, and the euglenids. It has also been reported from bacteria of the genus Thermus and also in Pyrococcus horikoshii, potentially suggesting a wider distribution than previously thought. This uniqueness of the pathway makes it a potentially interesting target for antimycotics.

L-Aspartic-4-semialdehyde is an α-amino acid derivative of aspartate. It is an important intermediate in the aspartate pathway, which is a metabolic pathway present in bacteria and plants. The aspartate pathway leads to the biosynthesis of a variety of amino acids from aspartate, including lysine, methionine, and threonine.

α-Aminoadipic acid is one of the metabolic precursor in the biosynthesis of lysine through α-aminoadipate pathway. Its conjugate base is α-aminoadipate, which is the prevalent form at physiological pH.


















