
Tricyclic antidepressants (TCAs) are a class of medications that are used primarily as antidepressants. TCAs were discovered in the early 1950s and were marketed later in the decade. They are named after their chemical structure, which contains three rings of atoms. Tetracyclic antidepressants (TeCAs), which contain four rings of atoms, are a closely related group of antidepressant compounds.

Chlorphenamine (CP, CPM), also known as chlorpheniramine, is an antihistamine used to treat the symptoms of allergic conditions such as allergic rhinitis (hay fever). It is taken orally (by mouth). The medication takes effect within two hours and lasts for about 4–6 hours. It is a first-generation antihistamine and works by blocking the histamine H1 receptor.

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
ATC code N06Psychoanaleptics is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup N06 is part of the anatomical group N Nervous system.

Flupentixol (INN), also known as flupenthixol, marketed under brand names such as Depixol and Fluanxol is a typical antipsychotic drug of the thioxanthene class. It was introduced in 1965 by Lundbeck. In addition to single drug preparations, it is also available as flupentixol/melitracen—a combination product containing both melitracen and flupentixol . Flupentixol is not approved for use in the United States. It is, however, approved for use in the UK, Australia, Canada, Russian Federation, South Africa, New Zealand, Philippines, Iran, Germany, and various other countries.

Pinazepam is a benzodiazepine drug. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties.

Melitracen is a tricyclic antidepressant (TCA), for the treatment of depression and anxiety. In addition to single drug preparations, it is also available as Deanxit, marketed by Lundbeck, a combination product containing both melitracen and flupentixol.

Nalorphine is a mixed opioid agonist–antagonist with opioid antagonist and analgesic properties. It was introduced in 1954 and was used as an antidote to reverse opioid overdose and in a challenge test to determine opioid dependence.

8-OH-DPAT is a research chemical of the aminotetralin chemical class which was developed in the 1980s and has been widely used to study the function of the 5-HT1A receptor. It was one of the first major 5-HT1A receptor full agonists to have been discovered.

Talopram, also known as phthalapromine, is a selective norepinephrine reuptake inhibitor (NRI) which was researched for the management of depression in the 1960s and 1970s but was never commercialized. Along with talsupram, talopram is structurally related to the selective serotonin reuptake inhibitor (SSRI) citalopram, as well as to melitracen:
In 1971, the company hired Klaus Bøgesø as a medicinal chemist. Over the years Bøgesø turned out to have a Midas touch at the game of drug hunting, creating more molecules that made it to the market than almost any other medicinal chemist in the field. The challenge facing him in 1971 following his recruitment was to produce a selective norepinephrine reuptake inhibitor. Like other companies at the time, Lundbeck had little interest in an SSRI. Bøgesø began from an accident in the laboratory. Trying to create a derivative of their norepinephrine reuptake inhibiting antidepressant melitracen, Lundbeck chemists accidentally produced a new chemical — a phenylphthalene. Against all the odds, just like melitracen, this was also a selective norepinephrine reuptake inhibitor. Two potential antidepressants came out of this — talopram and tasulopram, which were pressed into clinical trials. Both however turned out to be energizing, and in a number of cases there were suicide attempts. The fact that there were suicide attempts appeared to confirm another proposal of Paul Kielholz, that activating antidepressants might lead to suicide. Lundbeck's experience suggested that norepinephrine reuptake inhibitors were likely to lead to just this problem. Lundbeck retreated, scared. If norepinephrine reuptake inhibitors were likely to trigger suicide, the greatest hazard of an antidepressant, then Kielholz's view suggested that an SSRI would be less likely to lead to suicide. Bøgesø's job was to see whether the new series of drugs could be converted into a series of SSRIs. Following a lead from Carlsson on how to do this, he converted talopram into citalopram, the most selective serotonin reuptake inhibitor to come to the market.

UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. It causes dopamine release in the brain and has a stimulant effect, as well as blocking the behavioural effects of cocaine. It may also serve as a 5-HT2A receptor agonist, based on animal studies. It was investigated in clinical trials for the treatment of schizophrenia, but unexpectedly caused symptoms to become worse.

Dimetacrine, also known as dimethacrine and acripramine, is a tricyclic antidepressant (TCA) used in Europe and formerly in Japan for the treatment of depression. It has imipramine-like effects; though, in a double-blind clinical trial against imipramine, dimetacrine was found to have lower efficacy in comparison and produced more weight loss and abnormal liver tests.
Flupentixol/melitracen is a combination of two psychoactive agents flupentixol and melitracen. It is designed for short term usage only. It is produced by Lundbeck.

Fluotracen (SKF-28,175) is a tricyclic drug which has both antidepressant and antipsychotic activity. This profile of effects is similar to that of related agents like amoxapine, loxapine, and trimipramine which may also be used in the treatment of both depression and psychosis. It was believed that such duality would be advantageous in the treatment of schizophrenia, as depression is often comorbid with the disorder and usual antipsychotics often worsen such symptoms. In any case, however, fluotracen was never marketed.

Teniloxazine, also known as sufoxazine and sulfoxazine, is a drug which is marketed in Japan. Though initially investigated as a neuroprotective and nootropic agent for the treatment of cerebrovascular insufficiency in the 1980s, it was ultimately developed and approved as an antidepressant instead. It acts as a potent norepinephrine reuptake inhibitor, with fair selectivity over the serotonin and dopamine transporters, and also behaves as an antagonist of the 5-HT2A receptor.

Amitriptyline/perphenazine is a formulation that contains the tricyclic antidepressant amitriptyline and the medium-potency typical (first-generation) antipsychotic, perphenazine. In the United States amitriptyline/perphenazine is marketed by Mylan Pharmaceuticals Inc. and Remedy Repack Inc.
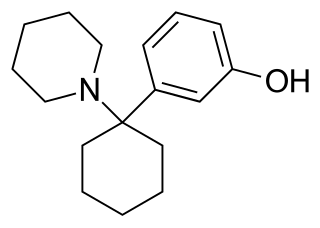
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug.
Tranylcypromine/trifluoperazine is a combination formulation of the monoamine oxidase inhibitor antidepressant drug tranylcypromine and the typical antipsychotic drug trifluoperazine that has been used in the treatment of major depressive disorder. It contains 10 mg tranylcypromine and 1 mg trifluoperazine. The drug has been in clinical use since at least 1961.

AC-90179 is a piperidine derivative which acts as an inverse agonist at the 5-HT2A serotonin receptor and an antagonist at 5-HT2C. It was developed as a potential antipsychotic but was not pursued for medical applications due to poor oral bioavailability; however, it continues to be used as a tool compound in pharmacological research.
















