
The hypothalamic–pituitary–adrenal axis is a complex set of direct influences and feedback interactions among three components: the hypothalamus, the pituitary gland, and the adrenal glands. These organs and their interactions constitute the HPA axis.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

Oxytocin is a peptide hormone and neuropeptide normally produced in the hypothalamus and released by the posterior pituitary. It plays a role in social bonding, reproduction, childbirth, and the period after childbirth. Oxytocin is released into the bloodstream as a hormone in response to sexual activity and during labour. It is also available in pharmaceutical form. In either form, oxytocin stimulates uterine contractions to speed up the process of childbirth. In its natural form, it also plays a role in bonding with the baby and milk production. Production and secretion of oxytocin is controlled by a positive feedback mechanism, where its initial release stimulates production and release of further oxytocin. For example, when oxytocin is released during a contraction of the uterus at the start of childbirth, this stimulates production and release of more oxytocin and an increase in the intensity and frequency of contractions. This process compounds in intensity and frequency and continues until the triggering activity ceases. A similar process takes place during lactation and during sexual activity.

The paraventricular nucleus is a nucleus in the hypothalamus. Anatomically, it is adjacent to the third ventricle and many of its neurons project to the posterior pituitary. These projecting neurons secrete oxytocin and a smaller amount of vasopressin, otherwise the nucleus also secretes corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH). CRH and TRH are secreted into the hypophyseal portal system and act on different targets neurons in the anterior pituitary. PVN is thought to mediate many diverse functions through these different hormones, including osmoregulation, appetite, and the response of the body to stress.
Corticotropes are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.
Dynorphins (Dyn) are a class of opioid peptides that arise from the precursor protein prodynorphin. When prodynorphin is cleaved during processing by proprotein convertase 2 (PC2), multiple active peptides are released: dynorphin A, dynorphin B, and α/β-neo-endorphin. Depolarization of a neuron containing prodynorphin stimulates PC2 processing, which occurs within synaptic vesicles in the presynaptic terminal. Occasionally, prodynorphin is not fully processed, leading to the release of “big dynorphin.” “Big Dynorphin” is a 32-amino acid molecule consisting of both dynorphin A and dynorphin B.

Agouti-related protein (AgRP), also called agouti-related peptide, is a neuropeptide produced in the brain by the AgRP/NPY neuron. It is synthesized in neuropeptide Y (NPY)-containing cell bodies located in the ventromedial part of the arcuate nucleus in the hypothalamus. AgRP is co-expressed with NPY and acts to increase appetite and decrease metabolism and energy expenditure. It is one of the most potent and long-lasting of appetite stimulators. In humans, the agouti-related peptide is encoded by the AGRP gene.

Vasopressin receptor 1A (V1AR), or arginine vasopressin receptor 1A is one of the three major receptor types for vasopressin, and is present throughout the brain, as well as in the periphery in the liver, kidney, and vasculature.

Vasopressin receptor 2 (V2R), or arginine vasopressin receptor 2, is a protein that acts as receptor for vasopressin. AVPR2 belongs to the subfamily of G-protein-coupled receptors. Its activity is mediated by the Gs type of G proteins, which stimulate adenylate cyclase.
The actions of vasopressin are mediated by stimulation of tissue-specific G protein-coupled receptors (GPCRs) called vasopressin receptors that are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. These three subtypes differ in localization, function and signal transduction mechanisms.
Vasotocin is an oligopeptide homologous to oxytocin and vasopressin found in all non-mammalian vertebrates and possibly in mammals during the fetal stage of development. Arginine vasotocin (AVT), a hormone produced by neurosecretory cells within the posterior pituitary gland (neurohypophysis) of the brain, is a major endocrine regulator of water balance and osmotic homoeostasis and is involved in social and sexual behavior in non-mammalian vertebrates. In mammals, it appears to have biological properties similar to those of oxytocin and vasopressin. It has been found to have effects on the regulation of REM sleep. Evidence for the existence of endogenous vasotocin in mammals is limited and no mammalian gene encoding vasotocin has been confirmed.

The oxytocin receptor, also known as OXTR, is a protein which functions as receptor for the hormone and neurotransmitter oxytocin. In humans, the oxytocin receptor is encoded by the OXTR gene which has been localized to human chromosome 3p25.

Neurophysin II is a carrier protein with a size of 19,687.3 Da and is made up of a dimer of two virtually identical chains of amino acids. Neurophysin II is a cleavage product of the AVP gene. It is a neurohypophysial hormone that is transported in vesicles with vasopressin, the other cleavage product, along axons, from magnocellular neurons of the hypothalamus to the posterior lobe of the pituitary. Although it is stored in neurosecretory granules with vasopressin and released with vasopressin into the bloodstream, its biological action is unclear. Neurophysin II is also known as a stimulator of prolactin secretion.

G protein-coupled estrogen receptor 1 (GPER), also known as G protein-coupled receptor 30 (GPR30), is a protein that in humans is encoded by the GPER gene. GPER binds to and is activated by the female sex hormone estradiol and is responsible for some of the rapid effects that estradiol has on cells.

Nelivaptan (INN) is a selective, orally active, non-peptide vasopressin receptor antagonist selective for the V1B subtype. The drug had entered clinical trials for treatment of anxiety and depression. In July 2008, Sanofi-Aventis announced that further development of this drug had been halted.
The Bruce effect, or pregnancy block, is the tendency for female rodents to terminate their pregnancies following exposure to the scent of an unfamiliar male. The effect was first noted in 1959 by Hilda M. Bruce, and has primarily been studied in laboratory mice. In mice, pregnancy can only be terminated prior to embryo implantation, but other species will interrupt even a late-term pregnancy.
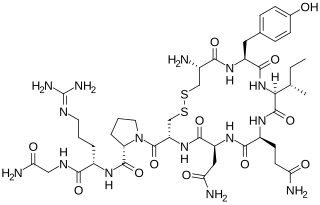
The neurohypophysial hormones form a family of structurally and functionally related peptide hormones. Their representatives in humans are oxytocin and vasopressin. They are named after the location of their release into the blood, the neurohypophysis.

Phenypressin (Phe2-Arg8-vasopressin) is an oxytocin neuropeptide belonging to the vertebrae vasopressin family and has similar pharmacological properties as arginine vasopressin. The name phenypressin came about because there is a substitution of phenylalanine that makes it different from arginine vasopressin in the second residue and that is the only difference. It belongs to the family, neurohypophysial hormones, named after the fact that they are secreted by the neurohypophysis which is a neural projection from the hypothalamus. It has mostly been found to be present is some species belonging to the family, Macropodidae, particularly eastern gray kangaroos[3], red kangaroos, tammar wallaby, and the quokka wallaby. In other marsupial families, Phenypressin has not yet specifically been identified, but they do have other vasopressin-like peptides present.
The biology of trust is the study of physiological mechanisms involved in mediating trust in social attachments. It has been studied in terms of genetics, endocrinology and neurobiology.

Arginine Vasopressin (AVP) Gene is a gene whose product is proteolytically cleaved to produce vasopressin, neurophysin II, and a glycoprotein called copeptin. AVP and other AVP-like peptides are found in mammals, as well as mollusks, arthropods, nematodes, and other invertebrate species. In humans, AVP is present on chromosome 20 and plays a role in homeostatic regulation. The products of AVP have many functions that include vasoconstriction, regulating the balance of water in the body, and regulating responses to stress. Expression of AVP is regulated by the Transcription Translation Feedback Loop (TTFL), which is an important part of the circadian system that controls the expression of clock genes. AVP has important implications in the medical field as its products have significant roles throughout body.


















