
Diabetes insipidus (DI), alternately called arginine vasopressin deficiency (AVP-D) or arginine vasopressin resistance (AVP-R), is a condition characterized by large amounts of dilute urine and increased thirst. The amount of urine produced can be nearly 20 liters per day. Reduction of fluid has little effect on the concentration of the urine. Complications may include dehydration or seizures.

Human vasopressin, also called antidiuretic hormone (ADH), arginine vasopressin (AVP) or argipressin, is a hormone synthesized from the AVP gene as a peptide prohormone in neurons in the hypothalamus, and is converted to AVP. It then travels down the axon terminating in the posterior pituitary, and is released from vesicles into the circulation in response to extracellular fluid hypertonicity (hyperosmolality). AVP has two primary functions. First, it increases the amount of solute-free water reabsorbed back into the circulation from the filtrate in the kidney tubules of the nephrons. Second, AVP constricts arterioles, which increases peripheral vascular resistance and raises arterial blood pressure.

Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer. Aquaporins have six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine–proline–alanine which form a barrel surrounding a central pore-like region that contains additional protein density. Because aquaporins are usually always open and are prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. Water can pass through the cell membrane through simple diffusion because it is a small molecule, and through osmosis, in cases where the concentration of water outside of the cell is greater than that of the inside. However, because water is a polar molecule this process of simple diffusion is relatively slow, and in tissues with high water permeability the majority of water passes through aquaporin.
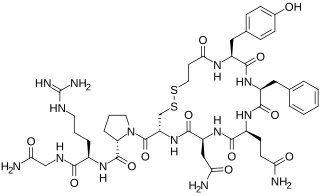
Desmopressin, sold under the trade name DDAVP among others, is a medication used to treat diabetes insipidus, bedwetting, hemophilia A, von Willebrand disease, and high blood urea levels. In hemophilia A and von Willebrand disease, it should only be used for mild to moderate cases. It may be given in the nose, by injection into a vein, by mouth, or under the tongue.
In biochemistry, in the biological context of organisms' regulation of gene expression and production of gene products, downregulation is the process by which a cell decreases the production and quantities of its cellular components, such as RNA and proteins, in response to an external stimulus. The complementary process that involves increase in quantities of cellular components is called upregulation.
Nephrogenic diabetes insipidus,, is a form of diabetes insipidus primarily due to pathology of the kidney. This is in contrast to central or neurogenic diabetes insipidus, which is caused by insufficient levels of vasopressin. Nephrogenic diabetes insipidus is caused by an improper response of the kidney to vasopressin, leading to a decrease in the ability of the kidney to concentrate the urine by removing free water.
The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone releasing hormone receptor (LHRHR), is a member of the seven-transmembrane, G-protein coupled receptor (GPCR) family. It is the receptor of gonadotropin-releasing hormone (GnRH). The GnRHR is expressed on the surface of pituitary gonadotrope cells as well as lymphocytes, breast, ovary, and prostate.

Vasopressin receptor 1A (V1AR), or arginine vasopressin receptor 1A is one of the three major receptor types for vasopressin, and is present throughout the brain, as well as in the periphery in the liver, kidney, and vasculature.
The actions of vasopressin are mediated by stimulation of tissue-specific G protein-coupled receptors (GPCRs) called vasopressin receptors that are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. These three subtypes differ in localization, function and signal transduction mechanisms.

Aquaporin-2 (AQP-2) is found in the apical cell membranes of the kidney's collecting duct principal cells and in intracellular vesicles located throughout the cell. It is encoded by the AQP2 gene.

The glucagon receptor is a 62 kDa protein that is activated by glucagon and is a member of the class B G-protein coupled family of receptors, coupled to G alpha i, Gs and to a lesser extent G alpha q. Stimulation of the receptor results in the activation of adenylate cyclase and phospholipase C and in increased levels of the secondary messengers intracellular cAMP and calcium. In humans, the glucagon receptor is encoded by the GCGR gene.

GNAS complex locus is a gene locus in humans. Its main product is the heterotrimeric G-protein alpha subunit Gs-α, a key component of G protein-coupled receptor-regulated adenylyl cyclase signal transduction pathways. GNAS stands for Guanine Nucleotide binding protein, Alpha Stimulating activity polypeptide.

DAX1 is a nuclear receptor protein that in humans is encoded by the NR0B1 gene. The NR0B1 gene is located on the short (p) arm of the X chromosome between bands Xp21.3 and Xp21.2, from base pair 30,082,120 to base pair 30,087,136.

The neuropeptide S receptor (NPSR) is a member of the G-protein coupled receptor superfamily of integral membrane proteins which binds neuropeptide S (NPS). It was formerly an orphan receptor, GPR154, until the discovery of neuropeptide S as the endogenous ligand. Increased expression of this gene in ciliated cells of the respiratory epithelium and in bronchial smooth muscle cells is associated with asthma. This gene is a member of the G protein-coupled receptor 1 family and encodes a plasma membrane protein. Mutations in this gene have also been associated with this disease.
Hepatocyte nuclear factor 4 alpha (HNF4A) also known as NR2A1 is a nuclear receptor that in humans is encoded by the HNF4A gene.

Cav2.1, also called the P/Q voltage-dependent calcium channel, is a calcium channel found mainly in the brain. Specifically, it is found on the presynaptic terminals of neurons in the brain and cerebellum. Cav2.1 plays an important role in controlling the release of neurotransmitters between neurons. It is composed of multiple subunits, including alpha-1, beta, alpha-2/delta, and gamma subunits. The alpha-1 subunit is the pore-forming subunit, meaning that the calcium ions flow through it. Different kinds of calcium channels have different isoforms (versions) of the alpha-1 subunit. Cav2.1 has the alpha-1A subunit, which is encoded by the CACNA1A gene. Mutations in CACNA1A have been associated with various neurologic disorders, including familial hemiplegic migraine, episodic ataxia type 2, and spinocerebellar ataxia type 6.

Endothelin receptor type B, (ET-B) is a protein that in humans is encoded by the EDNRB gene.

Melatonin receptor 1B, also known as MTNR1B, is a protein that in humans is encoded by the MTNR1B gene.

Wolframin is a protein that in humans is encoded by the WFS1 gene.
A vasopressin receptor antagonist (VRA) is an agent that interferes with action at the vasopressin receptors. Most commonly VRAs are used in the treatment of hyponatremia, especially in patients with congestive heart failure, liver cirrhosis or SIADH.

















