 | |
 | |
| Names | |
|---|---|
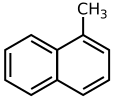
| Preferred IUPAC name 1-Methylnaphthalene | |
| Other names α-methylnaphthalene | |
| Identifiers | |
3D model (JSmol) | |
| 506793 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.788 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3082 1993 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C11H10 | |
| Molar mass | 142.20 g/mol |
| Appearance | Liquid |
| Density | 1.001 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 244.8 °C (472.6 °F; 518.0 K) [2] |
| Vapor pressure | 4.91 |
| −102.8·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H302, H304, H411 | |
| P264, P270, P273, P301+P310, P301+P312, P330, P331, P391, P405, P501 | |
| Flash point | 82 °C (180 °F; 355 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1-Methylnaphthalene is an organic compound with the formula C11H10. It is a colorless liquid. It is isomeric with 2-methylnaphthalene. It is generally obtained as a minor component of coal tar. [3]