| Clostridium difficile | |
|---|---|
 | |
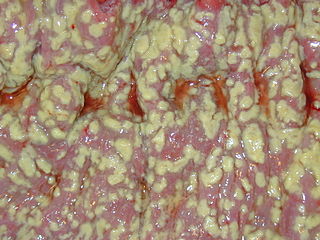
| C. difficile colonies on a blood agar plate | |
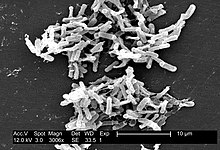 | |
| Micrograph of Clostridium difficile | |
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | Firmicutes |
| Class: | Clostridia |
| Order: | Clostridiales |
| Family: | Peptostreptococcaceae |
| Genus: | Clostridioides |
| Species: | C. difficile |
| Binomial name | |
| Clostridioides difficile Hall & O'Toole, 1935; Lawson & Rainey, 2016 | |
Clostridium difficile (etymology and pronunciation), also known as C. difficile, C. diff ( /siː
In biology, a species ( ) is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. A species is often defined as the largest group of organisms in which any two individuals of the appropriate sexes or mating types can produce fertile offspring, typically by sexual reproduction. Other ways of defining species include their karyotype, DNA sequence, morphology, behaviour or ecological niche. In addition, paleontologists use the concept of the chronospecies since fossil reproduction cannot be examined. While these definitions may seem adequate, when looked at more closely they represent problematic species concepts. For example, the boundaries between closely related species become unclear with hybridisation, in a species complex of hundreds of similar microspecies, and in a ring species. Also, among organisms that reproduce only asexually, the concept of a reproductive species breaks down, and each clone is potentially a microspecies.
Contents
- Renaming and scientific reclassification
- Human pathogen
- Transmission
- Host range
- Signs and symptoms
- Treatment
- Strains
- Genome
- Bacteriophage
- Naming and pronunciation
- Notes
- References
Clostridia (members of the genus Clostridium and of the Clostridiaceae family) are anaerobic, motile bacteria, ubiquitous in nature, and especially prevalent in soil. Its vegetative cells are rod shaped, pleomorphic, and occur in pairs or short chains. Under the microscope, they appear as long, irregular (often drumstick- or spindle-shaped) cells with a bulge at their terminal ends (forms subterminal spores). Under Gram staining, C. difficile cells are Gram-positive and show optimum growth on blood agar at human body temperatures in the absence of oxygen. C. difficile is catalase and superoxide dismutase negative, and produces two types of toxins: enterotoxin A and cytotoxin B, which disrupts cytoskeleton signal transductions in the host. When stressed, the bacteria produce spores that are able to tolerate extreme conditions that the active bacteria cannot tolerate. [2]

Clostridium is a genus of Gram-positive bacteria, which includes several significant human pathogens, including the causative agent of botulism. The genus formerly included an important cause of diarrhea, Clostridium difficile, which was separated after 16S rRNA analysis. They are obligate anaerobes capable of producing endospores. The normal, reproducing cells of Clostridium, called the vegetative form, are rod-shaped, which gives them their name, from the Greek κλωστήρ or spindle. Clostridium endospores have a distinct bowling pin or bottle shape, distinguishing them from other bacterial endospores, which are usually ovoid in shape. Clostridium species inhabit soils and the intestinal tract of animals, including humans. Clostridium is a normal inhabitant of the healthy lower reproductive tract of women.
An anaerobic organism or anaerobe is any organism that does not require oxygen for growth. It may react negatively or even die if free oxygen is present.

Motility is the ability of an organism to move independently, using metabolic energy. This is in contrast to mobility, which describes the ability of an object to be moved. Motility is genetically determined, but may be affected by environmental factors. For instance, muscles give animals motility but the consumption of hydrogen cyanide would adversely affect muscle physiology, causing them to stiffen, leading to rigor mortis. In addition to animal locomotion, most animals are motile – the term applies to bacteria and other microorganisms, and to some multicellular organisms, as well as to some mechanisms of fluid flow in multicellular organs and tissue. Motile marine animals are commonly called free-swimming, and motile non-parasitic organisms are called free-living.
C. difficile may become established in the human colon; it is present in 2–5% of the adult population. [2] Sometimes antibiotic therapy for various infections has the adverse effect of disrupting the normal balance of the gut microbiota, in which case C. difficile may opportunistically dominate, causing Clostridium difficile infection.

The large intestine, also known as the large bowel, is the last part of the gastrointestinal tract and of the digestive system in vertebrates. Water is absorbed here and the remaining waste material is stored as feces before being removed by defecation.

Infection is the invasion of an organism's body tissues by disease-causing agents, their multiplication, and the reaction of host tissues to the infectious agents and the toxins they produce. Infectious disease, also known as transmissible disease or communicable disease, is illness resulting from an infection.
In medicine, an adverse effect is an undesired harmful effect resulting from a medication or other intervention such as surgery.